Key Points:
- A newly developed CD38-targeting vaccine improves glucose levels and insulin signaling in aged mice.
- The CD38 vaccine also improves physical parameters, such as grip strength, and counteracts cognitive decline.
- At the cellular level, the CD38 vaccine elevates NAD+ levels and reduces age-promoting senescent cells.
What if we could slow down the aging process, or even reverse some of its effects? This is the tantalizing question that drives a significant amount of scientific research, and recent findings, published in Aging Cell, offer a glimmer of hope in the form of a novel vaccine.
A New Way to Combat Aging with NAD+
At the heart of the new findings lies NAD+ (nicotinamide adenine dinucleotide), a molecule crucial for energy production, DNA repair, and maintaining overall cellular health. Unfortunately, as we age, our NAD+ levels naturally decline. This drop is believed to contribute significantly to the aging process, leading to various age-related health issues.
One of the key culprits behind age-related NAD+ decline is an enzyme called CD38, which consumes NAD+ and effectively reduces its availability in our cells. While CD38 plays important roles in the immune system, its overexpression with age contributes to the depletion of NAD+, accelerating the aging process.
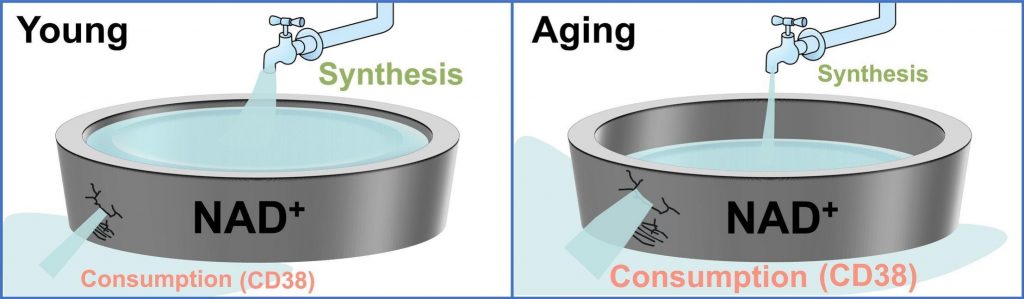
Scientists have explored various strategies for boosting NAD+, including supplements like NMN (nicotinamide mononucleotide) and NR (nicotinamide riboside). However, preventing elevations in CD38 may have similar, if not more potent, effects. In the Aging Cell study, researchers explored the potential anti-aging effects of a CD38-targeting vaccine. The findings suggest the vaccine restores NAD+ levels and, in turn, combats the effects of aging.
CD38 Vaccine Prevents Signs of Aging
The researchers focused on a specific peptide vaccine designed to target and eliminate immune cells expressing high levels of CD38. They aimed to see if reducing CD38 could lead to an increase in NAD+ levels and, consequently, an improvement in various aging-related conditions. The results were promising, showing that the CD38 vaccine not only elevated NAD+ levels but also led to several positive changes in aged mice.
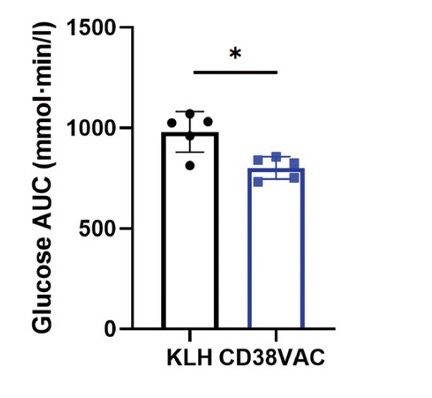
Improved Metabolic Health
Insulin is a hormone that signals our cells to intake glucose to make energy. However, with age, our cells become less sensitive to insulin and are unable to efficiently take up glucose, leading to high blood glucose levels. This lack of responsiveness to insulin is called insulin resistance, which increases the risk of age-related disorders, including neurodegenerative disorders, cardiovascular disease, and type 2 diabetes.
In vaccinated mice, the researchers observed better glucose tolerance and insulin sensitivity, suggesting the CD38 vaccine mitigates insulin resistance. Moreover, in a metabolic chamber, the vaccinated mice consumed more oxygen and produced more carbon dioxide, indicating improved metabolism and utilization of energy. Overall, these findings suggest that the CD38 vaccine ameliorates age-related metabolic decline, which could reduce the risk of several age-related disorders.
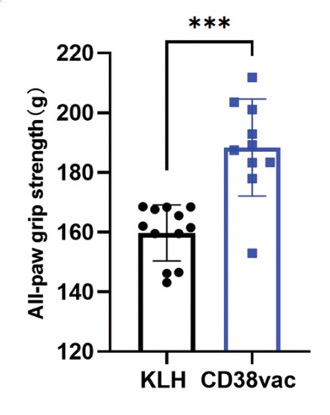
Enhanced Physical and Cognitive Performance
Sarcopenia is an age-related disorder, characterized by loss of muscle mass and strength, that predisposes people to premature death. Sarcopenia patients can find it difficult to perform basic movements like balancing and walking. The researchers found that vaccinated mice exhibited improved physical capabilities, including better performance in tests measuring walking distance, balance, grip strength, and endurance. This suggests a reversal or slowing of age-related physical decline that may potentially reduce the risk of sarcopenia and falls.
The researchers also observed improvements in cognitive function, with vaccinated mice performing better in memory and learning tasks. This is a crucial finding, as such a vaccine could potentially mitigate the prevalence of cognitive decline and dementia. Studies have estimated that two out of three Americans experience cognitive impairment by the age of 70, and up to 37% may experience dementia by the age of 80.
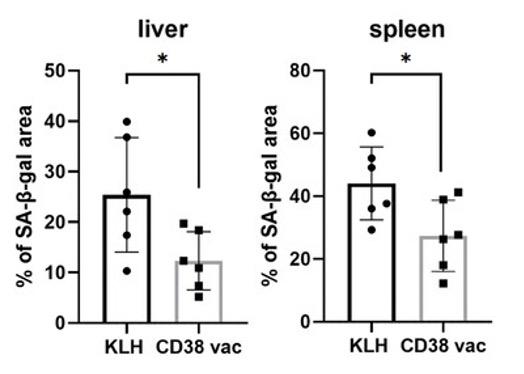
Reduction in Senescent Cells
In response to stressors like DNA damage and inflammation, our cells enter a senescent state. In our younger years, these senescent cells, which contribute to the prevention of cancer growth, are removed by the immune system. However, as our immune system weakens with age, senescent cells begin to accumulate. Since senescent cells secrete pro-inflammatory and organ-damaging molecules, they are thought to be an underlying driver of the aging process. Remarkably, in the liver and spleen of vaccinated mice, senescent cell numbers were reduced, suggesting the CD38 vaccine counteracts one of the root causes of aging.
Targeting CD38 without a Vaccine
While these results are exciting, it’s important to remember that this research was conducted on mice. The leap from mouse studies to human applications is a significant one, and much more research is needed before a CD38-targeting vaccine could be considered for human use. However, this study provides compelling evidence that targeting CD38 is a promising avenue for future anti-aging therapies.
Other compounds that inhibit CD38 and elevate NAD+ levels include the naturally occurring molecules apigenin, found in chamomile, and cyanidin-3-O-glucoside, found in elderberries. These molecules can be found in supplement form, but the dosage necessary to adequately raise NAD+ levels will need human studies. With that being said, human trials are underway testing FDA-approved CD38 inhibitors, like Darzalex and Sarclisa, against multiple myeloma, a white blood cell cancer. However, there is currently a lack of clinical trials testing the effect of CD38 inhibition against age-related disorders.