- Introduction: What are senolytics?
- Examples of senolytics and their current data
- Active and Planned Senolytic Human Trials
- Commercially available senolytics
As we grow older, the number of aged, non-proliferative senescent cells that release a milieu of inflammatory molecules to surrounding cells increases throughout our bodies. The molecule combination that senescent cells release (the senescence-associated secretory phenotype [SASP]) also induces healthy cells that come into contact with them to become senescent. Although most cells reach a point where they undergo programmed cell death (apoptosis), as we get older, more and more cells become senescent and survive molecular apoptosis cues. Since our immunity wanes with age, our immune systems also have a more difficult time disposing of senescent cells.
This age-associated accumulation of senescent cells facilitates tissue inflammation and organ damage. Intriguingly, some researchers propose age-related senescent cell accumulation is one of the reasons we age.
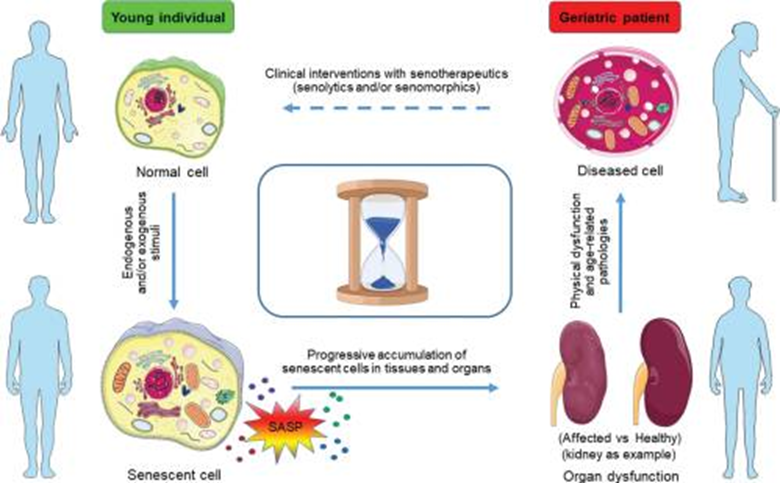
Senolytics Explained
Scientists have considered eliminating senescent cells as a way to counter the adverse effects of aging, namely to reduce inflammation and improve tissue function. For this purpose, researchers have recently developed a new class of supplements called senolytics, the majority of which are derived from a class of molecules found in fruits and vegetables called polyphenols. These new drugs can selectively trigger cell death (apoptosis) in senescent cells without considerably damaging healthy cells.
Senolytics work by targeting pro-survival pathways, which senescent cells use to evade apoptosis. A big challenge to eliminating senescent cells is that there are multiple types of these cells coming from different tissues, which rely on different pathways for apoptosis evasion. For this reason, the use of multiple senolytics, each targeting different pathways, may be necessary to terminate large swaths of senescent cells. Furthermore, some senescent cells play essential roles in wound healing, so figuring out what each senescent cell population does is critical before deciding which to target.
Examples of Senolytics and Their Preclinical Trials
Within the last decade, researchers have begun putting more stock into the idea that getting rid of senescent cells can counter some of aging’s adverse effects. Along those lines, a number of rodent studies have given positive results, propelling enthusiasm for senolytics.
Examples include the use of the senolytic agent quercetin, which, when combined with the chemotherapeutic agent dasatinib, was found to improve cardiac function in naturally aged and atherosclerosis mouse models. Another study using dasatinib and quercetin found the senolytic combination enhanced exercise capacity and radiation-damaged mice. The dasatinib and quercetin senolytic combination also delayed the onset of physical dysfunction in prematurely aging mice. These studies are among some of the major findings from animal studies using the senolytic agents dasatinib and quercetin.
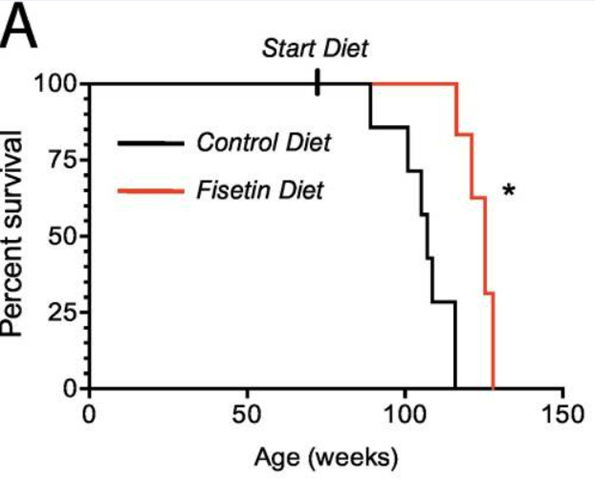
Fisetin, a plant flavonoid found in foods like strawberries and onions, has garnered positive findings when applied in mouse studies. Fisetin attenuates age-related pathologies and increases healthy mouse average lifespan by ~20%. The question remains how well the dasatinib and quercetin along with fisetin findings in rodent studies will translate to humans.
Yet another promising senolytic agent, procyanidin C1 (PCC1), is a plant-derived molecule found in grapes, unripe apples, and cinnamon. Intriguingly, a study has shown that supplementing aged, 24-month-old mice with PCC1 increased their remaining lifespan by close to 64%. PCC1 hasn’t garnered the attention which fisetin and quercetin have received, so future research should investigate the molecule’s pro-longevity potential.
Active and Planned Senolytic Human Trials
Data from early pilot human trials indicate that senolytics potently eliminate senescent cells, alleviate inflammation and reduce frailty in humans. Clinical trials for Alzheimer’s disease, COVID-19, diabetes, eye disorders, lung fibrosis, osteoarthritis, osteoporosis, bone marrow transplantation, and childhood cancer survivors are currently underway. Until the conclusion of such studies, it’s too early to say how effective senolytics will be for aged people.
The first senolytic clinical trial demonstrated improved physical function in patients with lung scarring (idiopathic pulmonary fibrosis) who took dasatinib and quercetin. Another human trial reported dasatinib and quercetin treatment reduces senescent cell burden in fat tissues (adipose tissue) of patients developing diabetic kidney disease. Moreover, circulating SASP factors inducing inflammation were reduced in these patients.
More recently, a phase I clinical trial of dasatinib and quercetin for Alzheimer’s disease reported that intermittent senolytic administration decreases a hallmark of Alzheimer’s disease pathology — tau protein aggregates. The same study showed dasatinib and quercetin reduce neuroinflammation, preserve neurons, and partly restore blood flow to the brain.
Given the promising findings associated with senolytics and their solid safety profiles, a number of new trials investigating their clinical potential have been initiated. Such studies with dasatinib and quercetin include those for mild cognitive impairment and Alzheimer’s disease, liver disease, and cancer. Fisetin is also being investigated for conditions like cancer, COVID-19, and Carpal Tunnel syndrome. If these trials produce positive results, using senolytic agents against age-related physiological decline may become more and more common.
Senolytics for Diabetes
Researchers recognize diabetes as one of the leading contributors to premature aging and the development of age-related conditions like cardiovascular disease and neurodegenerative disorders. Cellular senescence is believed to play a crucial role in developing insulin resistance and other diabetes-associated complications. Moreover, when an individual has diabetes, this can precipitate increased senescent cell formation. Along those lines, continuous exposure to high lipids and blood sugar (glucose) levels, both risk factors for diabetes, can trigger cellular senescence. Once senescent cells form, they release SASP factors to surrounding cells and tissue, triggering more cells to enter senscence and increasing the risk for diabetes. As such, using senolytics may help prevent the onset of diabetes.
Senolytics for Lung Fibrosis
A small clinical study conducted in 2014 revealed that patients with a lung tissue scarring condition called idiopathic pulmonary fibrosis experienced physical functioning improvements following treatment with dasatinib and quercetin, two senolytics. These results show promise for senolytics improving lung health since dasatinib and quercetin can potentially treat this lung disease. These results require future studies using larger study groups for confirmation.
Senolytics for Kidney Disease
Patients with diabetes-related kidney disease showed improved physical functioning following senolytic consumption. A Mayo Clinic study has shown that dasatinib and quercetin treatment reduced senescent cells from the fatty tissue of kidney disease patients with diabetes. Furthermore, circulating SASP factors that inflame nearby cells and tissues were reduced following dasatinib and quercetin therapy. These findings draw us one step closer to confirming that rodent study data showing senolytics alleviate age-related diseases can apply to humans.
Senolytics for Osteoarthritis
Osteoarthritis is an age-related disease where patients lose joint cartilage, which can debilitate them, leaving them unable to physically function. Individuals with osteoarthritis complain of extreme pain when walking, adversely affecting mobility. By not walking so much due to pain, aged individuals with this condition can abstain from physical exertion, furthering their physical debilitation. Along those lines, finding effective ways to treat this condition is paramount.
Recently, researchers have shown that cartilage-generating cells, chondrocytes, can become senescent, emitting SASP factors to surrounding cells and joints. Targeting these cells with senolytics could offer an effective way to treat osteoarthritis. Promisingly, one recent study showed that the senolytic ABT-263 can promote the function of cartilage-producing stem cells taken from osteoarthritis patients. Future studies will need to determine whether this senolytic compound applied to osteoarthritis patients improves their physical function.
Senolytics for Alzheimer’s Disease
Alzheimer’s disease, an age-related condition marked by progressive cognitive decline, includes the accumulation of amyloid-beta plaques in the brain. Interestingly, protein aggregates in senescent cells include amyloid-beta plaques. Thus, curbing senescent cell accumulations with senolytics could be a way to mitigate Alzheimer’s-related pathology.
One of the cells composing the brain’s barrier between the blood and the brain (the blood-brain barrier) are astrocytes. Astrocytes have a number of roles for brain protection aside from the blood-brain barrier as well. When astrocytes become senescent, they release copious amounts of SASP factors to surrounding cells and tissues, limiting the clearance of amyloid-beta plaques. This scenario leads to increased amyloid-beta plaque levels and facilitates worsening of Alzheimer’s disease symptoms.
The senolytic combination of dasatinib and quercetin has been found in a small clinical trial to alleviate the accumulation of SASP factors, easing the spread of senescence to surrounding cells. Dasatinib and quercetin could therefore alleviate Alzheimer’s disease by suppressing the spread of SASP factors but will need future clinical trials for confirmation.
Senolytics for Cancer
An estimated 17 million people have cancer in the US, causing about 9.6 million deaths annually. Cancer starts with a single cell that acquires a mutation increasing its ability to divide. With these cancer mutations, cells can indefinitely divide and proliferate. Cancer cells also compete with other nearby healthy cells for nutrients. Cancer cells thereby outcompete healthy cells for nutrients, causing them to outnumber healthy cells and for the cancer to grow.
When cancer cells become senescent, their dividing and proliferation capabilities decline. Thus, when senescent, their abilities to increase in abundance and migrate to other parts of the body diminish. Additionally, the SASP factors that cancer cells release would have anti-cancer effects on other cancer cells in their vicinity. For these reasons, some researchers believe that inducing senescence in cancer cells can arrest their growth and spread.
A promising senolytic drug that induces senescence in cancer cells is Palbociclib, which selectively inhibits proteins allowing the proliferation of cancer cells. Furthermore, the inhibition of these proteins leads to cancer cell growth arrest and senescence. Palbociclib has shown promising findings for breast cancer, melanoma, gastric cancer, hepatocellular carcinoma, and liposarcoma.
While the effects of senescent cell accumulation in the body as we get older is under investigation, the evidence showing this accumulation plays a key role in aging makes it a promising target for anti-aging drugs. As such, senolytics present a potential new option to selectively eliminate senescent cells for inflammation reduction and improving tissue health.
| Senolytic | Findings | Rodent Model | Dietary Sources |
|---|---|---|---|
| Fisetin | When administered beginning at 85 weeks of age, fisetin extended mouse median lifespan by about 20% | X | Strawberries, apples, onions, grapes, kiwi |
| Quercetin | Mouse median lifespan increased by about 6.3% with quercetin and dasatinib treatments starting between 24 and 27 months of age | X | Capers, onions, kale, okra, apple peels |
| Curcumin | Mice taking curcumin starting at 13 weeks of age exhibited a lifespan increase by about 10% | X | Turmeric |
| Procyanidin C1 (PCC1) | Aged, 24-month-old mice treated with PCC1 showed 64.2% longer remaining lifespan | X | Grapes, unripe apples, cinnamon |