Key Points:
- Anti-aging therapies that may help alleviate or prevent cardiovascular problems include those targeting metabolism, such as calorie restriction and NAD+ precursors.
- Other anti-aging therapies for the heart, such as rapamycin, target the enhancement of autophagy, a cellular mechanism by which cells recycle and degrade their own components.
- Additionally, anti-aging therapies that promote heart function, such as senolytic compounds, selectively eliminate cells that have reached a permanent state of growth arrest and can also release inflammatory molecules.
As the global population shifts towards an aged populace, a phenomenon referred to as the “silver tsunami,” so do the medical and socioeconomic burdens of heart disease. As such, as published in Cardiovascular Research, Kroemer and colleagues from Sorbonne University in France propose that aging is one of, if not the, critical risk factor for the development of heart disease. However, there are currently no approved therapeutics primarily targeting the effects of aging processes on the heart.
This publication from the French research group presents emerging anti-aging strategies, which may delay or reverse the development of age-related cardiovascular conditions. Such anti-aging interventions target metabolism, autophagy (the cell’s recycling and waste disposal mechanism), and the elimination of dysfunctional cells that accumulate with age, called senescent cells. Along those lines, these anti-aging compounds may serve to counteract several prevalent age-related heart conditions like atherosclerosis, hypertension, and heart failure. The overarching goal of outlining these anti-aging therapies is to challenge the notion that aging is a non-modifiable risk factor for cardiovascular disease.
Background on How Aging Affects the Heart
The heart has the essential function of distributing vital nutrients throughout the body and eliminating waste. As such, the cardiovascular system operates under a tremendous amount of shear stress and exposure to biochemical waste from the entire body. Notably, heart muscle cells, responsible for the heart’s pumping action, have a limited proliferative capacity, meaning they cannot be easily replaced and must survive for most of the human lifespan. All of these factors make aging one of, if not the, major risk factor for cardiovascular disease since aging entails the gradual decline in function of the cardiovascular system and other physiological processes.
Anti-Aging Therapeutics for the Heart that Modify Metabolism
An increased incidence of obesity with age, compounded with other factors like low physical activity, smoking, and psychological stress, can exacerbate the dysregulation of metabolism. Critically, dysregulated metabolism has been directly tied to an increased risk of heart disease. In line with this notion, a failing heart’s dysregulated metabolic processes can lead to heart failure. Importantly, anti-aging strategies targeting metabolic function could help to preserve cardiovascular function and prevent age-related heart disease.
Calorie Restriction
One such anti-aging intervention strategy for metabolic modulation is calorie restriction, a dietary pattern that involves consuming significantly fewer calories than usual without becoming malnourished. Calorie restriction is one of the most studied metabolism-modifying strategies, with ample data suggesting it has body-wide rejuvenative effects, including within the cardiovascular system. Indeed, calorie restriction without malnutrition has been shown to extend lifespan in multiple organisms, from mice to monkeys, possibly through positive effects on cardiovascular function.
Regarding the effects of calorie restriction on the cardiovascular system, research has demonstrated that this dietary pattern protects the vasculature against age-related stiffening and enhances vascular dilation in humans and mice, respectively. Both cardiovascular benefits, protection against vascular stiffening and enhancement of dilation, lower the risk of cardiovascular disease. At the cellular level, these cardiovascular benefits are intricately tied to reduced signaling of mTOR, a protein with a central role in regulating cell growth and metabolism.
Remarkably, in non-obese individuals, calorie restriction lowers blood pressure, reduces harmful LDL cholesterol, and reduces the 10-year risk for cardiovascular disease onset by 30%. Consequently, a calorie-restricted diet represents a potential metabolism-modifying strategy to mitigate and perhaps even treat age-related cardiovascular conditions.
NAD+ Precursors
While calorie-restricted dieting may be too difficult for some people to regularly engage in, other metabolism-modifying therapeutics may work against age-related cardiovascular disease. These therapeutics include nicotinamide adenine dinucleotide (NAD+) precursors like nicotinamide mononucleotide (NMN), which studies have shown increase circulating NAD+ in humans.
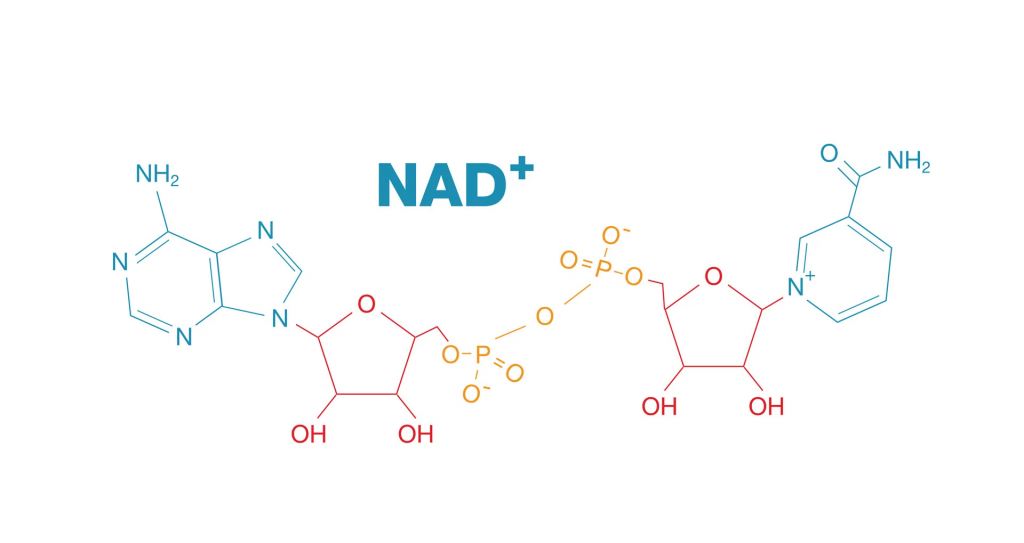
Cellular NAD+ levels have been shown to decline with age in humans. As such, reduced cellular NAD+ with age can trigger several dysfunctional attributes in cells relevant to cardiovascular problems, such as altered nutrient sensing and increased inflammation. Also, declining NAD+ levels have been associated with several age-related cardiovascular risk factors, such as obesity and high blood pressure. Thus, targeting low NAD+ levels with NAD+ precursors has gained attention for the potential to treat age-related cardiovascular conditions, including atherosclerosis and heart failure.
In support of this notion, NAD+ supplementation using its precursor nicotinamide demonstrated effects in preventing age-related heart dysfunction in male mice. Furthermore, another study showed that replenishing NAD+ with its precursors enhanced vascular elasticity and vascular dilation in aged male mice. These cardiovascular benefits were accompanied by body-wide metabolic reprogramming, characterized by a shift toward improved bioenergetics—biochemical processes that produce and use energy.
In humans, replenishing NAD+ with the precursor nicotinamide riboside (NR) has shown promise in lowering blood pressure in middle-aged and elderly adults. NR has also been shown to lower inflammation and improve energy production in the cell’s powerhouse (the mitochondria) in humans. Consequently, these results warrant larger human trials to establish NAD+ precursors as safe and effective therapies modulating metabolism to prevent or treat age-related cardiovascular conditions, along with other age-related diseases.
Anti-Aging Therapeutics for Autophagy Activation
Throughout the body, maintaining cellular function requires tight regulation through a network of mechanisms involving cellular machinery. Chief among these is a cellular process called autophagy, through which cells recycle and degrade their dysfunctional components, such as damaged proteins, and dispose of debris. Notably, the activation and efficiency of this cellular process deteriorate with age in the heart and vasculature.
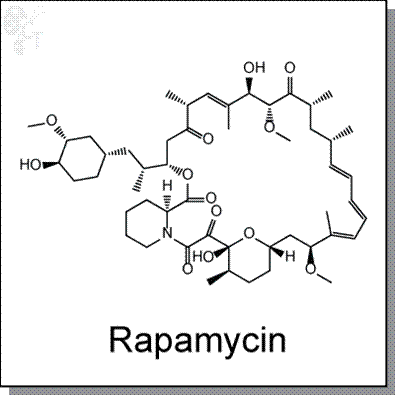
Accordingly, this age-related declining function of autophagy contributes to declining cardiovascular health and the associated rise in heart disease risk. Supporting this notion, dysfunctional autophagy exists in multiple age-related cardiovascular diseases, including atherosclerosis, coronary heart disease, and heart failure. Importantly, autophagy-activating compounds like rapamycin and its analogs inhibit mTOR to induce this cellular function-promoting process.
Interestingly, rapamycin has been shown to extend lifespan across multiple species, from worms to mice. Rapamycin’s lifespan extension effects in mice may be due, in part, to its cardiac function-improving properties. Whether autophagy activators like rapamycin improve cardiac function and slow cardiovascular aging in humans will require future clinical trials for testing.
Anti-Aging Therapeutics that Selectively Eliminate Senescent Cells
Senescence is a cellular state of permanent cessation of growth and proliferation, and cellular senescence is a key hallmark of aging. Once in a state of senescence, cells can become resistant to cell death and release inflammatory molecules that induce senescence in surrounding cells, thereby increasing inflammation and perpetuating age-related senescence in surrounding tissue.
Scientists have proposed that the accumulation of senescent cells in the cardiovascular system may contribute to cardiac aging and related cardiovascular diseases through the loss of normal cells and the release of inflammatory molecules. Accordingly, the selective elimination of senescent cells using compounds called senolytics may represent a promising way to counteract cardiovascular aging and age-related cardiovascular diseases, like atherosclerosis, high blood pressure, and heart failure.
Remarkably, in preclinical research, aged mice with blocked blood flow to the heart showed enhanced cardiac functional recovery, increased heart regenerative potential, and higher survival in response to senolytic therapy. Also, in a model of cardiac aging that consisted of a cluster of human cardiac cells, senolytic agents rejuvenated these cells. All the same, future human trials will be necessary to more definitively assess whether senolytic agents protect against an age-related decline in cardiac function.
Targeting Multiple Facets of Cardiovascular Aging
The promise of slowing cardiovascular aging with anti-aging therapeutics that modulate metabolism, activate autophagy, and selectively eliminate senescent cells may serve to ease the medical and socioeconomic burden of an aging global populace. Interestingly, some aging intervention products, like Restorin, contain multiple components targeting all three of these aspects of cardiovascular aging. For example, Restorin’s NAD+ precursor technology may modulate metabolism, and the product also contains autophagy-activating and senolytic technologies.
Thus, if the preclinical data using animal models for these components translates to humans, aging intervention products may already be available that simultaneously target each of these aspects of cardiovascular aging. Only future clinical trials testing functional parameters of the cardiovascular system can help determine if such products containing combinations of technologies counteract cardiovascular aging.