Key Points:
- Increasing COX7RP gene activity extends lifespan in male mice.
- COX7RP improves how mitochondria produce energy, increasing ATP while lowering damaging oxidative stress in fat and muscle.
- In aged fat tissue, COX7RP reduces inflammatory aging signals, shifting cells away from a senescent, pro-aging state.
Aging disrupts the body’s ability to produce energy efficiently and increases chronic inflammation. As mitochondria — the cell’s energy producers — become less efficient with age, cellular energy output declines while harmful byproducts known as reactive oxygen species accumulate beyond the cell’s ability to neutralize them. These byproducts damage cells and accelerate aging. Over time, damaged cells begin releasing inflammatory signals that further impair tissues, especially fat tissue, which becomes increasingly dysfunctional with age. Because few interventions have extended lifespan under normal conditions, scientists have begun focusing on improving mitochondrial efficiency itself as a way to slow aging.
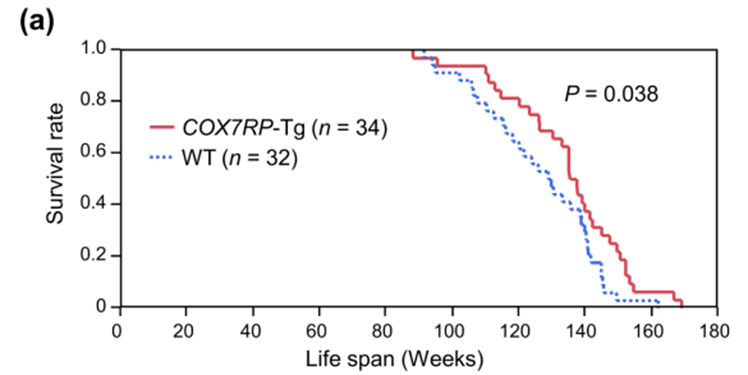
In a new study published in Aging Cell, Ikeda and colleagues tested whether strengthening the internal organization of mitochondria could slow aging across the whole body. The researchers used genetically engineered mice that produce extra amounts of COX7RP, a protein that helps mitochondria arrange their energy-producing machinery more efficiently. The team found that boosting COX7RP extended lifespan in male mice and produced a broad anti-aging profile marked by improved metabolism, higher cellular energy reserves, lower oxidative stress, fewer aging cells, and reduced inflammatory activity in older fat tissue.
COX7RP Extends Lifespan and Reshapes White Fat
The most direct outcome was survival. Male mice with higher COX7RP levels lived significantly longer than normal mice. Average lifespan increased by 6.6%, while median lifespan increased by 5.6%. Female mice showed a similar upward trend, though it was not strong enough to be statistically conclusive.
Importantly, this lifespan extension did not come from drastic weight loss or altered feeding behavior. Overall body weight and most organ weights remained similar between groups. Instead, the difference was localized to white fat tissue. At 10 months of age, mice with higher COX7RP had significantly less visceral and subcutaneous fat — types of fat closely linked to insulin resistance, inflammation, and age-related metabolic disease.
COX7RP Improves Blood Fats and Blood Sugar Control
To see whether reduced fat mass translated into better metabolic health, the researchers examined blood lipid levels and performed standard metabolic tests. Mice with higher COX7RP had lower triglyceride and cholesterol levels, both of which are linked to cardiovascular risk.
Blood sugar testing showed a modest but consistent improvement. After consuming glucose, COX7RP mice cleared sugar from their blood more efficiently, returning to normal levels faster than control mice. Insulin levels also tended to be lower, suggesting the body was using insulin more effectively. Additional testing showed no change in how the liver produces glucose, indicating that COX7RP primarily improves insulin sensitivity rather than suppressing energy production.
COX7RP Improves Cellular Energy While Reducing Cellular Damage
COX7RP does not directly create energy. Instead, it helps mitochondria organize their energy-producing components into tightly connected structures called respiratory supercomplexes. These structures allow electrons to flow more efficiently during energy production, reducing energy loss and limiting damage.
In older mice, fat and muscle tissue with higher COX7RP showed stronger mitochondrial performance. These tissues consumed more oxygen — a sign of higher energy production — and produced more ATP, the molecule cells use as fuel. At the same time, mitochondria generated fewer reactive oxygen species, which can be harmful and damage proteins, DNA, and cell membranes.
Levels of NAD⁺, a molecule that supports energy metabolism and cellular repair, were also higher. NAD⁺ naturally declines with age, and restoring it is considered beneficial for longevity.
Crucially, these improvements came from better organization of existing mitochondrial machinery rather than simply increasing the amount of mitochondrial proteins. The mitochondria were working smarter, not harder.
![COX7RP Overexpression Increases Cellular Energy Production and Reduces Senescence Markers. Compared to wild-type (WT) mice, COX7RP transgenic (COX7RP-Tg) mice exhibited significantly higher ATP synthesis rates in white adipose tissue [WAT (fat tissue)] and quadriceps muscle, indicating enhanced mitochondrial energy production. In parallel, senescence-associated β-galactosidase (SA-β-Gal) activity, a marker of cellular aging, was significantly reduced in WAT from COX7RP-Tg mice.](https://cms.nad.com/wp-content/uploads/2025/12/image-2-1024x414.png)
COX7RP Reduces Inflammatory Aging Signals in Fat Cells
The most striking aging-specific effect appeared in white fat tissue. Using single-cell analysis, the researchers examined how aging changed gene activity in individual fat cells. In older normal mice, fat cells showed increased activity of genes involved in the senescence-associated secretory phenotype, or SASP — inflammatory and tissue-damaging molecules.
In contrast, aged fat cells from COX7RP mice showed much lower activity of these inflammatory SASPs. Collectively, key inflammatory signals were reduced, indicating that fewer fat cells had entered a senescent, pro-aging state.
This shift was supported by additional tests showing lower activity of classic inflammatory molecules and reduced levels of senescence-associated β-galactosidase, a commonly used marker of aging cells. Together, the data suggest that improving mitochondrial efficiency helps prevent fat cells from becoming inflammatory drivers of aging.
COX7RP’s Potential as an Anti-Aging Strategy
Taken together, the study connects mitochondrial organization to lifespan in a clear and biologically coherent way. By improving how mitochondria generate energy, COX7RP increases cellular fuel availability, lowers damaging oxidative stress, preserves NAD⁺ levels, and reduces the accumulation of inflammatory aging cells — particularly in fat tissue, a key regulator of metabolic health during aging.
This work does not yet represent a ready-to-use therapy. The intervention is genetic rather than drug-based, and the findings are limited to mice. However, the results point to a promising target. If future treatments can safely enhance COX7RP activity or improve mitochondrial organization in humans, they may be able to slow multiple aging processes at once — improving metabolism, reducing inflammation, and extending healthspan.