Key Points:
- The study ties certain mitochondrial DNA sequences, inherited from one’s mother, with the length of telomeres—the protective DNA sequences at the ends of chromosomes.
- Infusing mitochondria-depleted human bone cancer cells with mitochondria from human donor platelets (to make cells called cybrids) confirmed a correlation between shorter telomeres and reduced mitochondrial function.
- Treating cybrids with NR and the antioxidant NAC protected against telomere shortening under conditions of cellular oxidative stress, high levels of damaging reactive molecules.
The shortening of the telomeres—the protective DNA sequences at the ends of chromosomes—happens as we get older, and this phenomenon constitutes a hallmark of aging. Accordingly, shortened telomeres have been linked to age-related diseases affecting organs like the lungs, kidneys, and liver.
Research suggests that genetic factors contribute between 36% to 82% of shortened telomeres, which may drive aging, in part, by causing cells throughout the body to enter a dysfunctional state (senescence). However, pinpointing exactly which genetic sequences are tied to shortened telomeres has remained a challenge.
Now, as published in a preprint study, Decottignies and colleagues from UCLouvain in Belgium show an association between genetic sequences from the cell’s powerhouse (mitochondria), which are inherited from one’s mother, and shorter telomere lengths. Also, taking mitochondrial DNA from donor blood platelets and infusing them into mitochondria-depleted human bone cancer cells (to make cells called cybrids) revealed that shorter telomeres were associated with higher levels of oxidative stress (cellular stress from high levels of damaging, reactive molecules), a sign of reduced mitochondrial function. Interestingly, treating the cybrid cells with the antioxidant NAC and the nicotinamide adenine dinucleotide (NAD+) precursor NR preserved telomere length under conditions that induced oxidative stress. These findings suggest that NAC combined with NR may help preserve telomere length, especially for those with inherited mitochondrial DNA sequences associated with shorter telomeres.
NR and NAC Combined Counteract Telomere Shortening
Everyone’s mitochondrial DNA comes almost exclusively from their mother—a concept called maternal inheritance. Moreover, previous evidence suggests that telomere length is strongly inherited from one’s mother. Given the association between telomere length being strongly influenced by maternal inheritance and mitochondrial DNA coming almost exclusively from one’s mother, Decottignies and colleagues sought to investigate how human mitochondrial DNA influences telomere length.
To this end, they analyzed the mitochondrial genetics and telomere lengths of seven individuals. Based on analyses of their telomeres using telomere fluorescent tags observed under a microscope, three of these people were classified as having long telomeres, two denoted as having average-length telomeres, and two dubbed as having short telomeres.
The researchers then examined certain mitochondrial genetic markers associated with individuals in the long, average, and short telomere groups. Along those lines, one of the individuals with long telomeres (above the 95th percentile for telomere length) had a specific mitochondrial genetic marker called A177T—a mitochondrial genetic variant associated with longevity and a protective effect against Parkinson’s disease. Intriguingly, the mother of this individual also had telomeres above the 90th percentile for telomere length. This data supports the idea of maternal inheritance of mitochondrial DNA influencing telomere length.
Adding to evidence for maternal inheritance of mitochondrial DNA modulating telomere length, another person assessed as having long telomeres had a mother and daughter with telomere lengths above the 90th percentile. In contrast, her father, whom she presumably did not inherit mitochondrial DNA from, had telomere lengths around the 50th percentile. Collectively, these findings support the notion of a maternal inheritance pattern of mitochondrial DNA and certain genetic variants in mitochondrial DNA influencing telomere length.
Mitochondrial DNA influences the function of mitochondria, and dysfunctional mitochondria impair telomere elongation in mice. Furthermore, this inhibition of telomere elongation is believed to arise from increased oxidative stress, an occurrence also tied to impaired mitochondrial function.
Thus, to assess whether mitochondrial DNA sequences from people with varying lengths of telomeres influence oxidative stress levels, Decottignies and colleagues measured oxidative stress in cybrids formed with mitochondrial DNA of participants with short, average, and long telomeres. These oxidative stress levels served as a proxy to assess mitochondrial function across cybrids with varying telomere lengths as well.
The researchers found that longer telomere length in the cybrids was associated with lower oxidative stress in cells. Relatedly, the researchers found an association between shorter telomeres and elevated cellular oxidative stress. Altogether, these findings support an association between mitochondrial function and telomere length, where shorter telomeres are associated with reduced mitochondrial function and higher oxidative stress.
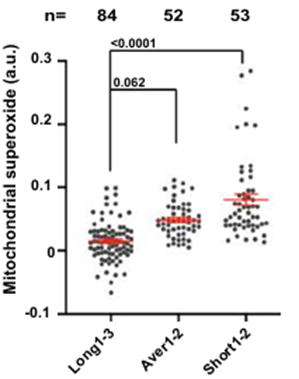
To pinpoint what mitochondrial DNA variants may contribute to shorter telomeres, Decottignies and colleagues assessed a subset of the cybrids with average and long telomeres, which, counterintuitively, showed elevated oxidative stress. As such, these cybrids did not follow the previous association where longer telomeres were associated with reduced oxidative stress. Interestingly, Decottignies and colleagues found that cybrids formed from these donors’ mitochondrial DNA had mutations in sequences involved in cell energy generation. Moreover, when these cybrids were exposed to cellular conditions that worsened oxidative stress, their telomeres shortened. These findings support the notion that certain inherited mitochondrial DNA sequences influence mitochondrial function (as measured with oxidative stress levels) and telomere length.
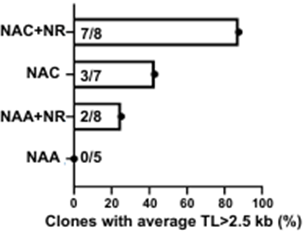
Building on this idea, Decottignies and colleagues used cybrids with mitochondrial DNA from one of the donors with average telomere length that showed the counterintuitive association with dysfunctional mitochondria. Because the NAD+ precursor NR has been shown to increase mitochondrial function, and since the antioxidant NAC helps against oxidative stress, the researchers treated these cybrids with these two compounds to find whether they protect against telomere shortening. Intriguingly, they found that the combination of these two compounds preserved telomere length under cellular conditions that induce oxidative stress. Moreover, either NR or NAC treatment alone also prevented telomere shortening, although not to the degree that the combination of these compounds did. These findings suggest that NR and NAC combined may preserve telomere length, especially in individuals with inherited mitochondrial DNA sequences that predispose to shortened telomeres.
The Possibility that NR and NAC Preserve Human Telomeres During Aging
The study from Decognitties and colleagues provides evidence that certain sequences in mitochondrial DNA, inherited maternally, may influence telomere length. The data also suggest that shortened telomeres arise from dysfunction of the cell’s mitochondria. Interestingly, treating cybrids carrying mitochondrial DNA mutations that were associated with mitochondrial dysfunction with NR and NAC combined helped preserve telomere length under telomere-shortening cellular conditions.
These findings raise the question of whether supplementing with NR and NAC can help preserve telomere length in adults, especially those with mitochondrial DNA sequences that may lower mitochondrial function. Along those lines, preserving telomere length could be important since shortened telomeres, which worsen with age, have been tied to age-related diseases affecting various organs. In that sense, research has already demonstrated an association between taking another NAD+ precursor, nicotinamide mononucleotide (NMN), and telomere extension in humans. Thus, while human trials are necessary to confirm that adults with mitochondrial DNA sequences that may inhibit mitochondrial function can use NR and NAC to preserve telomere length, the possibility for these two compounds to aid telomere preservation to counteract aging remains an open question.