Key Points:
- CD38 immune cells are elevated in individuals predisposed to Alzheimer’s disease.
- Inhibiting CD38 improves the cognition and metabolic fitness of Alzheimer’s mice.
- CD38 inhibition mitigates animal-modeled Alzheimer’s disease by reducing brain inflammation.
Brain aging is intricately linked to immune system aging, whereby a compromised immune system disrupts brain function, leading to cognitive decline and neurodegenerative diseases like Alzheimer’s disease (AD). However, about 30 years ago, making this link amongst neuroscientists and immunologists would have had you laughed out of the room.
It wasn’t until 1998 that Dr. Michal Shwartz broke scientific dogma by pioneering research showing that the immune system plays a critical role in protecting and repairing the brain. Now that the link between immunity and the brain is well established, Shwartz and her colleagues have continued to make advancements, focusing on finding treatments for age-related brain diseases like AD.
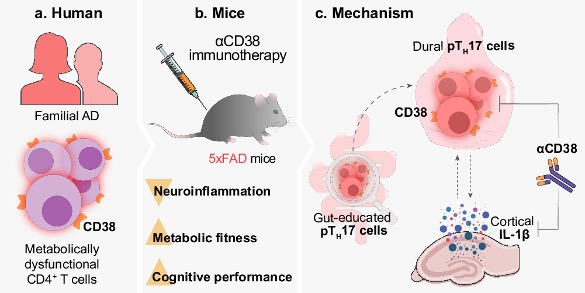
In a study published in Nature Communications, Shwartz and colleagues show that individuals predisposed to AD have more immune cells harboring the cell surface enzyme CD38. CD38 consumes NAD+ (nicotinamide adenine dinucleotide) and is thought to be a major contributor to age-related NAD+ decline. The researchers found that blocking CD38 improves the cognition and metabolic fitness of AD mice. If more progress is made in this arena, these findings may lead to new AD therapies involving CD38 inhibition.
CD38 Is Elevated in Pre-Symptomatic Alzheimer’s Individuals
Highlighting how little we know of AD etiology, over 90% of AD cases are sporadic, meaning the cause is unknown. Only 5 to 10% of AD cases are genetic, a form of AD called familial AD. Familial AD has traditionally been linked to mutations in genes such as APP (amyloid precursor protein). However, researchers recently discovered individuals from an ethnic group in Israel with a form of familial AD linked to having two copies of APP.
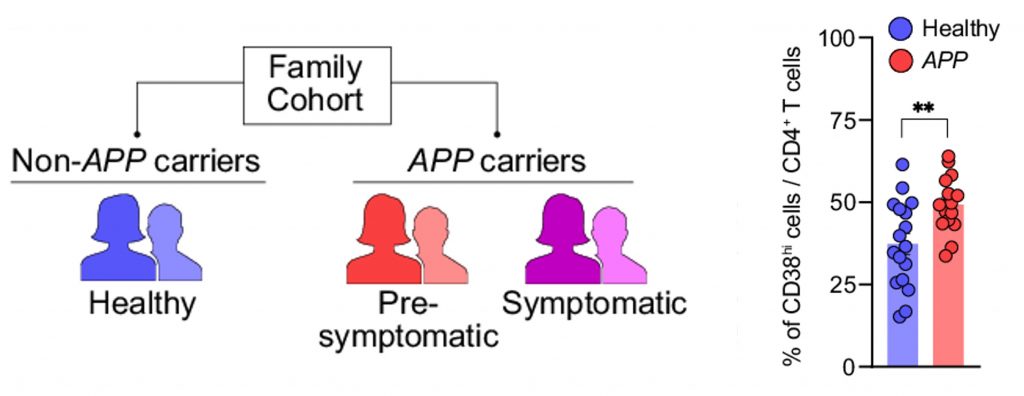
A family of these individuals who agreed to participate in the study was examined by Shwartz and colleagues to determine the relevance of CD38 in humans. The participants lacking two APP copies were designated as healthy and compared to participants carrying the APP duplication. The APP duplication carriers were further divided based on whether they experienced clinical symptoms of AD:
- Healthy participants do not carry two copies of APP.
- Pre-symptomatic participants carry two copies of APP but do not experience symptoms of AD.
- Symptomatic participants carry two copies of APP and experience symptoms of AD.
Upon finding that there were only four symptomatic carriers, the researchers decided to analyze only the healthy and pre-symptomatic participants. As such, CD38-expressing immune cells were measured from blood samples taken from the healthy and pre-symptomatic participants. The results showed that, compared to the healthy particpants, the pre-symptomatic participants had significantly higher levels of CD38 expressing immune cells.
The CD38-expressing immune cells in question are a type of white blood cell known as helper T cells. There are multiple types of T cells, called so because they are generated in the thymus. Helper T cells get their name from helping other immune cells fight off antigens. They express a surface protein called CD4, making them CD4-positive (CD4+) T cells. CD4 is a receptor that allows helper T cells to present antigens to other immune cells.
Shwartz and colleagues found that the CD4+ T cells in pre-symptomatic patients were dysfunctional in multiple ways. Aside from expressing higher levels of CD38, they exhibited defects in glucose utilization. Dysfunctional mitochondria were also observed in the CD4+ T cells of pre-symptomatic patients. Together, the findings suggest that CD38-expressing helper T cells could be a target for new AD therapies.
Inhibiting CD38 Counteracts Memory Loss and Neuroinflammation
To further examine the role of CD38 in AD, Shwartz and her team used the 5xFAD mouse model for AD. These mice harbor five AD-linked genetic mutations found in familial AD patients, hence the name 5xFAD (5x Familial Alzheimer’s disease). 5xFAD mice are widely used by scientists and model an early and aggressive form of the disease.
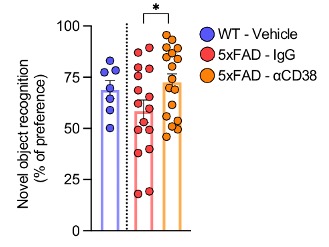
To see if higher CD38 expression contributes to AD, the researchers injected 5xFAD mice with an antibody that inhibits CD38. By measuring parameters such as food intake and energy expenditure, they found that inhibiting CD38 improves the metabolic fitness of 5xFAD mice. Moreover, inhibiting CD38 was shown to improve the cognition of the 5xFAD mice. Cognition was assessed with the novel object recognition (NOR) test, which exploits the tendency of mice to spend more time exploring novel objects over familiar ones.
The key mechanism by which immune system aging contributes to brain aging is through chronic low-grade inflammation. When our immune cells stop functioning properly, they erroneously recruit pro-inflammatory proteins to healthy organs, degenerating them over time. The dysfunction of our organs is what causes age-related diseases, like AD, and eventually death.
As a proxy for brain inflammation, Shwartz and colleagues measured a pro-inflammatory protein called IL-1β. They found that IL-1β was elevated in 5xFAD mouse brains but reduced by CD38 inhibition, suggesting that inhibiting CD38 counteracts neuroinflammation. Together, these findings suggest that inhibiting CD38 counteracts the cognitive dysfunction associated with AD by mitigating metabolic defects and brain inflammation.
The Role of the Gut
To explore how inhibiting CD38 can lead to the symptoms and pathology of AD, the researchers examined a subset of CD4+ T cells called Th17 cells, which secrete the pro-inflammatory protein IL-17. The pathogenic version of Th17 cells, called pathogenic Th17 cells, are associated with autoimmune diseases. Shwartz and colleagues found that pathogenic Th17 cells were more abundant in the brains of 5xFAD mice and could be diminished by inhibiting CD38. Moreover, further experiments revealed that IL-1β stimulates an increase in pathogenic Th17 cells, suggesting that inhibiting CD38 deters a sequence of inflammatory events.
Th17 cells, which usually play a protective role against pathogens in the gut, can be found within the intestines. The researchers showed that, compared to normal mice, the 5xFAD mice had higher levels of intestinal pathogenic Th17 cells. Based on these results, the authors speculate that the Th17 cells become pathogenic in the gut, leading to pathogenic Th17 cells in the brain via the gut-brain axis. Elevated IL-1β levels in the brain may also contribute to pathogenic Th17 cells. Thus, inhibiting CD38 appears to reduce neuroinflammation by disrupting pathogenic Th17 cell signaling.
Can Boosting NAD+ Have Similar Effects to Inhibiting CD38?
We have previously reported on a study showing that inhibiting CD38 with a drug called 78c increases the lifespan of mice by 10% while increasing NAD+ levels in multiple organs and improving physical performance. Moreover, the NAD+ precursors NR (nicotinamide riboside) and NMN (nicotinamide mononucleotide) have been shown to increase the lifespan of mice by 5% and 8.5%, respectively. Thus, boosting NAD+ and inhibiting CD38 both have pro-longevity effects.
However, combining a NAD+ repletion with CD38 inhibition may be the best option, as NR has been shown to enhance the effects of CD38 inhibition. Moreover, Shwartz and colleagues demonstrate that the mechanism leading to reduced neuroinflammation and the mitigation of AD symptoms by CD38 inhibition does not necessarily involve NAD+. Nevertheless, more studies are needed to determine the precise mechanism by which CD38 reduces brain inflammation in 5xFAD mice.
In humans, when combined with other anti-aging compounds, NR has been shown to improve the cognitive function of AD patients. An interesting next step would be to give AD patients both a NAD+ boosting and CD38 inhibiting compound. For example, AD patients could be given a combination of the NAD+-boosting NR and apigenin, a plant-based compound shown to inhibit CD38 and enhance the cognition of elderly mice.