Key Points:
- Cholesterol buildup in a type of white blood cell — macrophages — triggers nicotinamide adenine dinucleotide (NAD+) depletion, propelling a senescent state.
- Senescent macrophages drive the progression of age-related macular degeneration (AMD) — an eye disease that is the leading cause of blindness after age 50 — in mice.
- The NAD+-boosting molecule nicotinamide mononucleotide (NMN) reverses senescence in macrophages and restores eye function in a mouse model of AMD.
Published in Cell Reports, Apte and colleagues from Washington University School of Medicine in St. Louis provide data suggesting that cellular NAD+ depletion leads to senescence, at least in white blood cells called macrophages in mice. They also show that, in macrophages, falling cellular NAD+ and senescence propel age-related macular degeneration (AMD) — a debilitating condition that can facilitate blindness — in mice. Furthermore, treating senescent macrophages with NMN reversed their senescence, and injecting AMD model mice with NMN restored their eye function. These findings suggest that NMN, on its own or added to medications used to prevent vision loss, works against AMD and may pave the way for clinical trials testing whether NMN gives the same benefits for humans.
Cholesterol Buildup within Macrophages Depletes NAD+ and Induces Age-Related Macular Degeneration
Apte and colleagues suspected that cholesterol buildup in macrophages leads to AMD. This is because a deficiency in a protein that traffics cholesterol out of macrophages called ABCA1 has been shown to induce AMD in mice. Moreover, genetic variations in the human ABCA1 gene have been associated with increased susceptibility to AMD. The Washington University researchers thus genetically engineered mice with impaired ABCA1 function, and as expected, these mice showed cholesterol accumulation in their macrophage immune cells.
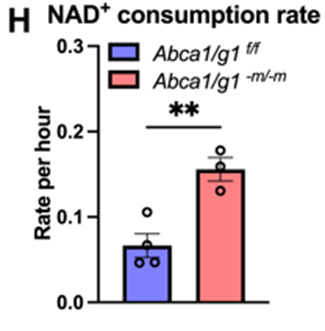
Not only that but macrophages from these mice showed substantial reductions in NAD+ and a near doubling of cellular NAD+ breakdown. To find out how this occurs, the researchers performed a gene activity analysis and discovered increased activation for a gene that produces the protein CD38 in response to increased cholesterol within cells.
CD38 helps traffic cholesterol out of macrophages via ABCA1. CD38 also consumes copious amounts of NAD+ to perform its functions. In this way, the data suggests that inhibiting cholesterol trafficking out of cells by reducing ABCA1 function increases cholesterol in macrophages. Elevated cholesterol then facilitates increased CD38 gene activation and subsequent NAD+ depletion.
Apte and colleagues then sought to find whether NAD+ depletion induces senescence — an aging-associated dysfunctional cellular state — in macrophages. Moreover, they sought to find out whether restoring NAD+ reverses senescence. This is important, because senescent macrophage accumulation has been linked to AMD. If NAD+ depletion was to facilitate their senescence, NAD+ restoration may be a way to reverse macrophage senescence and treat AMD.
They found that inhibiting NAD+ biosynthesis, in fact, increases the activation of genes associated with senescence — p16 and p21 — in macrophages from healthy mice. Furthermore, NMN treatment suppressed senescence in macrophages extracted from a mouse model for AMD. According to the authors of the study, these findings suggest that NAD+ repletion with NMN reverses senescence in macrophages affected by AMD.
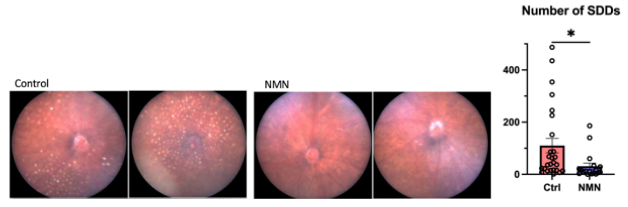
Apte and colleagues also found that the AMD model mice displayed yellow-white deposits within the eye called subretinal drusenoid deposits (SDDs). These deposits are physiological markers of AMD. Furthermore, treating the AMD model mice with drugs that selectively eliminate senescence (dasatinib and quercetin) effectively suppressed SDD formation. Dasatinib and quercetin also improved measurements of eye health, such as reducing the thickening of a membrane within the eye that occurs with AMD called Bruch’s membrane thickening. These findings corroborate that senescent macrophage accumulation within the eye drives AMD.
Since NMN was shown to reverse macrophage senescence — which is tied to AMD — in lab dishes, Apte and colleagues sought to find whether the compound also suppresses SDD formation and improves faltering eye function, both of which are signatures of AMD. They found that NMN injections suppressed the abundance of SDDs in the AMD model mice.
Moreover, they performed an electroretinogram — a test that measures the electrical activity of light-sensitive neurons within the eye (photoreceptors) when stimulated with light. This test serves as an indicator of eye function. The electroretinogram showed that NMN improved photoreceptor responses to light in the AMD model mice. These findings support that NMN reduces burdensome SDDs within the eye and improves eye function in AMD, possibly through suppressing or reversing macrophage senescence.
“In summary, our findings uncover [cholesterol trafficking] as a converging mechanism underlying senescence induction and that NAD+ augmentation is an effective approach to reverse macrophage senescence and age-related diseases,” say Apte and colleagues.
NMN May Also Reverse Senescence and/or Eliminate Senescent Cells In Other Tissues
The noteworthy findings that NMN seemed to reverse senescence in macrophages from AMD model mice may help explain why this compound has other benefits like improving physical function and insulin sensitivity. Although this study only examined NMN’s potential anti-senescence effects in macrophage cells, the possibility looms that the compound has similar benefits against senescence in other cell types. If NMN eliminates senescent cells or reverses senescence in other cell types, this would help explain this compound’s broad spectrum of benefits on the cardiovascular, neurological, and muscular systems.
Since senescent cell accumulation is one of the hallmarks of aging — meaning that it occurs because of aging or may partially cause aging — eliminating or reversing senescence may be a crucial way to counteract age-related physiological decline. In that sense, the finding that NMN may work against cellular senescence could bring us one step closer to unraveling how it confers its wide array of physiological benefits. With that said, we need further experimentation to find whether NMN confers anti-senescence benefits in other cell types.
Future clinical trials are also required to find whether NMN’s effects against AMD in mice translate to people. If NMN is, in fact, found to counteract AMD in people, using this compound in addition to currently prescribed medications may improve the chances of preserving vision for patients with AMD.