Key Points:
- Brief treatment with nicotinamide and EGCG restored neurons’ energy within 16–24 hours, restarted their internal cleanup systems, and reduced buildup of Alzheimer-related proteins.
- The combination increased neuron survival by about 22% in the most stressed cultures and improved several aging-linked measures, suggesting a practical way to help keep brain cells working with age.
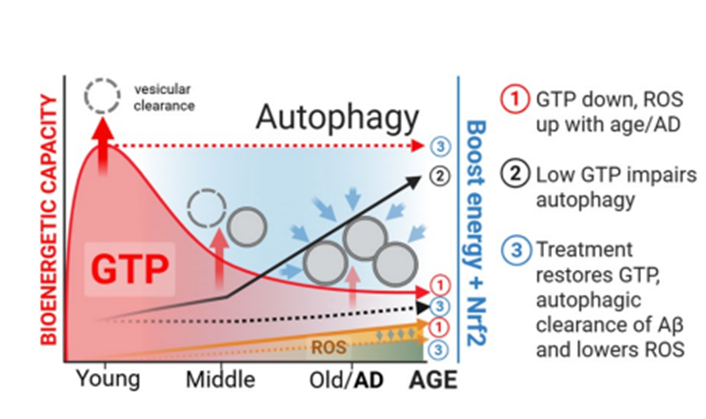
As brains get older, nerve cells can run low on GTP (guanosine triphosphate), a small energy molecule that powers cargo handling and recycling inside cells. When that fuel thins out, two housekeeping jobs slow down: endocytosis (when cells take items in to sort them, like a mailroom) and autophagy (when cells recycle worn-out parts, like a repair shop). A new study in GeroScience shows that topping up aging neurons with nicotinamide (a vitamin B3 form) and EGCG (the well-known green-tea compound) restores GTP levels and gets those cleanup systems moving again in lab models of aging and Alzheimer’s disease.
What Changes with Age
The team cultured mouse hippocampal neurons, including cells from the 3xTg-AD strain that develops Alzheimer-like changes, and tracked GTP inside mitochondria with a fluorescent biosensor. As neurons aged, mitochondrial GTP levels declined, and autophagy slowed down. These signs of reduced autophagy were most pronounced in the Alzheimer-model neurons.
To test whether autophagy draws from reserves of GTP, the researchers nudged it in opposite directions. Stimulating autophagy with rapamycin resulted in a decrease in GTP, consistent with the autophagy process consuming GTP to use as energy. In contrast, blocking autophagy caused GTP to rise. These findings link the GTP pool to powering cellular housekeeping.
A Simple Rescue
Next came the rescue step. Neurons received nicotinamide, which replenishes NAD⁺ to support energy metabolism and GTP synthesis, together with EGCG, an activator of the Nrf2 transcriptional program that boosts antioxidant defenses and stabilizes redox homeostasis.
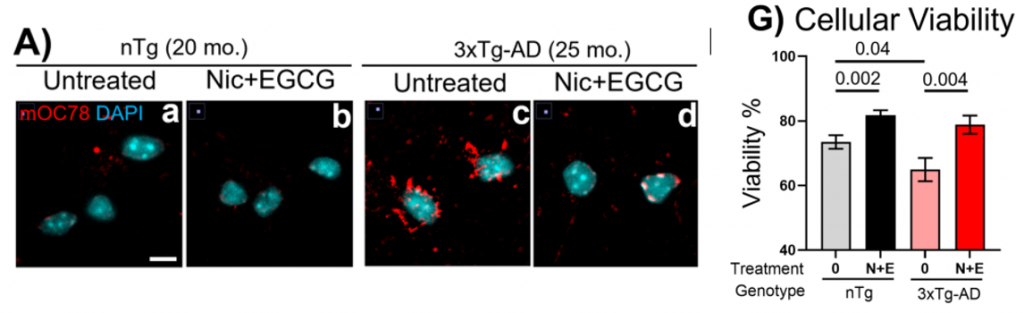
Within 16–24 hours, mitochondrial/GTP recovered to youthful levels; enlarged vesicles contracted, and endo-lysosomal trafficking – the route that moves cargo from intake vesicles toward lysosomes, the cell’s acidic recycling bins – restarted. In 3xTg-AD neurons, intracellular amyloid-beta (Aβ) burden declined, and markers of protein oxidative/nitrative damage decreased. Neuron viability increased by ~22% under the most stressful condition. Each monotherapy produced partial benefits, while the combination delivered a broader rescue consistent with simultaneous fuel repletion and redox control.
Why This Matters for Aging
Neurons are long-lived. They cannot rely on frequent replacement, so their survival hinges on steady, low-drama maintenance. Aging appears to chip away at the energy budget that keeps that maintenance going. When neurons come up short on GTP, routine tasks – sorting, shuttling, and recycling – slow down, leading to clutter and protein accumulation. The study’s rescue shows that a modest, targeted push can restore capacity: top up NAD⁺ with nicotinamide to feed core metabolism, use EGCG to flip on protective genes and balance redox chemistry, and the cleanup crew gets moving again.
The benefits center on classic features of brain aging: stalled autophagy, lysosomal backlog, oxidative stress, mitochondrial strain, and amyloid-beta accumulation. Seeing all of these move in a better direction at once strengthens the case that bioenergetic shortfalls sit near the root of the problem. It also outlines a testable strategy for healthy brain aging: support the fuel supply and the quality-control systems may keep pace longer.
Where This Could Go Next
These are dish-grown mouse neurons, not patients. The next steps are straightforward to imagine: studies in living animals to map dose and timing, checks for cognitive benefits, and long-term safety work. The tools themselves are familiar. Nicotinamide is widely used to bolster NAD⁺, and EGCG is a well-studied dietary compound. That familiarity makes it easier to design careful experiments that look for durable gains in neuronal housekeeping, fewer toxic aggregates, and preserved function with age.
A Working Model for Neuronal Maintenance
Taken together, the data outline a clear picture: age-related drops in mitochondrial GTP coincide with stalled trafficking and slower lysosomal turnover, and brief support with nicotinamide plus EGCG restores the energy pool, normalizes routing, lowers intracellular amyloid-beta, and improves neuron survival in stressed cultures. The mechanism is straightforward (more available fuel and steadier redox control), and the response spans several hallmarks of brain aging.
Translation now depends on studies in living animals. Showing the same rescue in aged animals, at doses that reach the brain and remain safe, would set the stage for trials that track memory and behavior alongside cellular readouts. If those results hold, maintaining the neuronal energy budget for routine housekeeping could become a practical way to preserve function later into life. For now, this work sharpens the focus on a basic requirement of healthy neurons: enough fuel for the cleanup crew to stay on schedule.