Key Points:
- White blood cells transfer DNA to other white blood cells, which then secrete rivers of DNA.
- The DNA rivers reduce senescent cells—cells associated with promoting biological aging—in multiple organs.
- The DNA rivers prolong the lifespan of mice by a duration roughly equivalent to 50 human years.
One of the earliest theories of aging revolves around telomeres—the repetitive sequences of DNA bookending chromosomes. Telomeres incrementally shorten each time our cells divide. And according to telomere theory, when our telomeres become too short, our cells stop dividing and eventually die, ultimately leading to the death of the entire organism.
However, as other factors besides telomere shortening contribute to organismal death, telomere theory has been largely disproven. Still, researchers from a biotechnology company called Sentcell and Oxford University may have just changed the way we think about telomeres. They have discovered that so-called “telomere Rivers” could be the answer to aging.
The Role of the Immune System
Telomere Rivers are generated by the immune system through the interaction between immune cells. To surmount an attack from foreign invaders, called antigens, our immune cells must extensively communicate. Immune cells called antigen-presenting cells (APCs) engulf antigens and present them to other immune cells, such as T cells. Once presented with a specific antigen, T cells can then destroy more of that antigen.
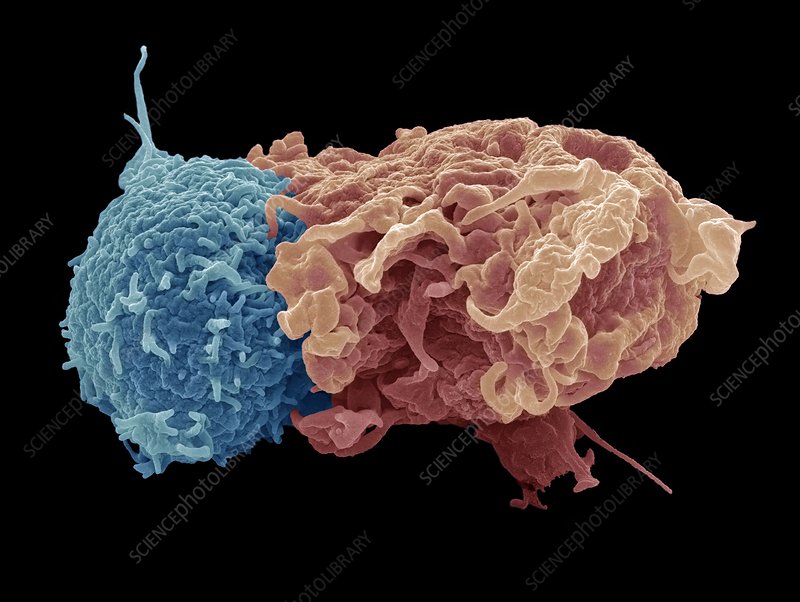
While the role of APCs and T cells in destroying antigens is well established, Sentcell researchers recently discovered that APCs can transfer telomeres to T cells. What’s more, the transferred telomeres fuse to and elongate the telomeres of receiving T cells, promoting T cell longevity. Thus, by donating telomeres, APCs are capable of rejuvenating T cells.
Discovering Telomere Rivers
In their new study with Oxford scientists, the Sentcell researchers further examined T cell dynamics. In doing so, they noticed genetic material surrounding T cells containing a transporter called CPT1A. CPT1A transports fat molecules into mitochondria, whereby the fat is metabolized into cellular energy, which can be utilized to sustain T cell longevity.
The genetic material observed outside of the CPT1A-equipped T cells was perplexing, as telomeres do not usually exist freely outside of cells. However, using special dyes, the researchers confirmed that the genetic material surrounding the T cells was telomeres. The researchers described the architecture of these telomeres and aptly named them, saying,
“Histology revealed that these extracellular telomeres were not merely tethered to T cells but arranged in vessel-like networks, suggesting release into circulation. The elongated, punctate structures appeared to flow along these networks, evoking miniature streams of genetic material — henceforth referred to as telomere Rivers.”
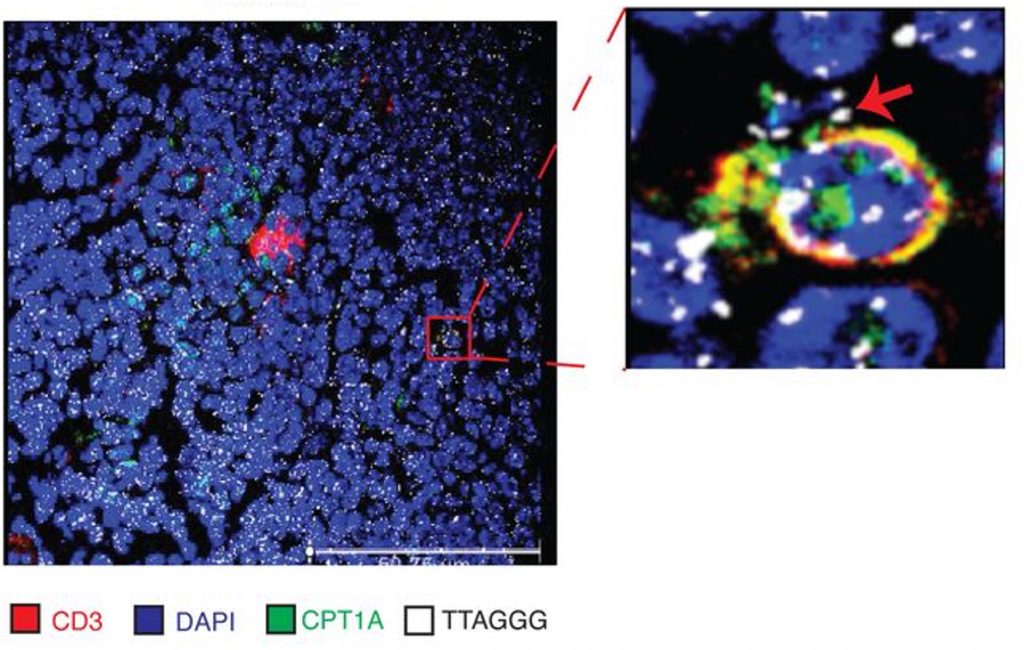
Subsequently, the researchers found telomere Rivers circulating in the blood of mice, and similar telomere structures were observed in human blood and plant sap, suggesting telomere Rivers are conserved across species. Interestingly, the plants required light (photosynthesis) to generate telomere Rivers. However, in darkness, the telomere Rivers could be induced in plants by genetically elevating CPT1A.
Further experiments revealed that CPT1A was necessary for APCs to transfer telomeres to T cells. Moreover, the telomere Rivers were only generated in response to antigens, whereby the APCs could interact with T cells to transfer the telomeres. The results suggest that excess transferred telomeres within T cells are then secreted into circulation. However, the precise purpose behind T cell telomere River secretion may be elucidated in future studies.
Telomere Rivers Reduce Cellular Senescence
Senescent cells are thought to drive the aging process. The majority of our cells are susceptible to becoming senescent cells, as long as they incur enough molecular damage, such as DNA damage from telomere shortening. Senescent cells fail to function normally and can promote tissue damage and aging by secreting tissue-degrading and pro-inflammatory molecules. Thus, getting rid of excess senescent cells can counteract the biological aging process.
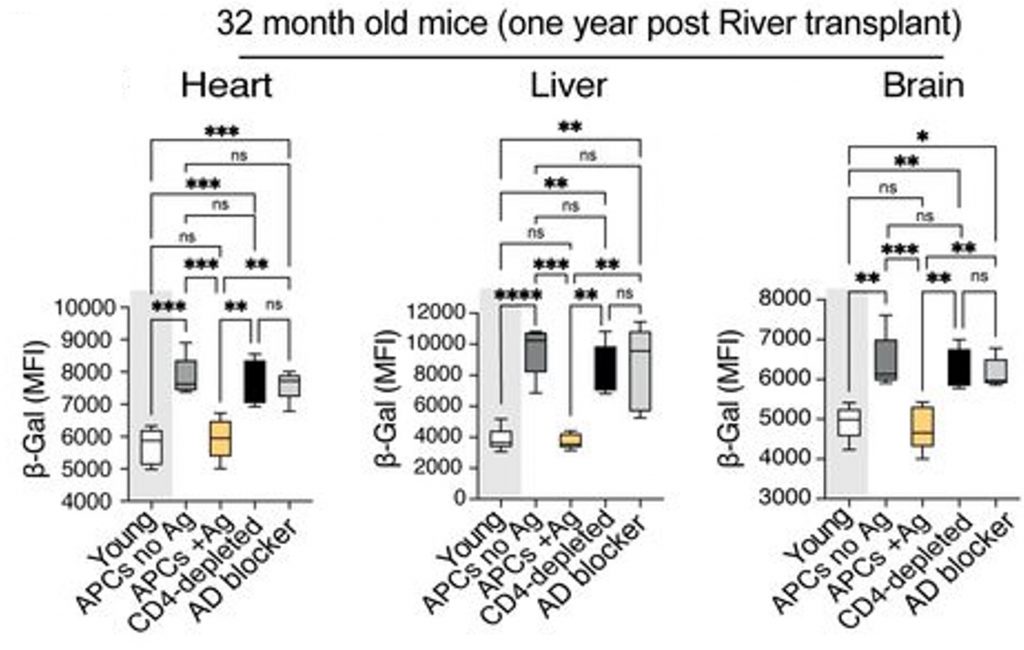
To determine if telomere Rivers can rejuvenate entire organisms, the researchers isolated telomere Rivers from young mice. They transferred the young telomere Rivers to 20-month-old mice, roughly equivalent in age to 60-year-old humans. One year later, the River-treated mice showed a reduction in senescent cells in multiple organs, including the heart, liver, and brain. This occurred only when APCs, T cells, and an antigen were present.
Telomere Rivers Prolong Lifespan
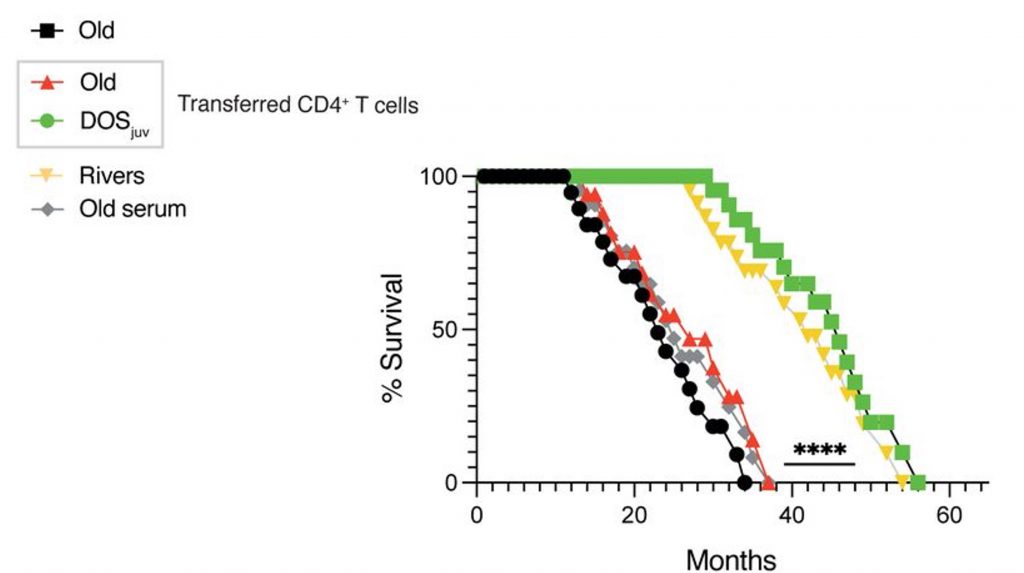
The researchers also tested whether they could prolong the lifespan of mice. In doing so, they experimented with five groups:
- Old: Old mice were not treated with anything and used to compare with the other groups.
- Old with transferred T cells: Old mice were treated with T cells from old mice.
- DOS (Disruptors of sestrin-MAPK interactions): Old mice were treated with rejuvenated T cells, whereby fat metabolism was restored by DOS.
- Rivers: Old mice were treated with telomere Rivers.
- Old serum: Old mice were treated with serum from old mice.
After treatment, the old mice were injected with a flu vaccine to elicit an immune reaction. The results showed that old mice treated with old T cells or old serum lived just as long as untreated old mice. Remarkably, however, the median lifespan of the old mice treated with rejuvenated T cells or telomere Rivers increased by a whopping 17 months, which is roughly equivalent to 54 human years. What’s more, some of the mice lived up to five years, which is more than double the normal lifespan of mice.
The Future of Rejuvenation Transplants
The researchers conclude,
“By tracing [telomere Rivers’] origin, composition, and systemic effects, we now define the first immune-regulated and transplantable programme of youth. The evolutionary conservation of Rivers across species—and even kingdoms—opens the prospect of engineering youth-promoting signals transferable between organisms, providing a conceptual framework for future immune-driven rejuvenation transplants.”
Still, more research is needed to determine precisely how telomere Rivers trigger a reduction in senescent cells and an extension in lifespan. Additionally, more tests will be needed to assess whether telomere Rivers reap adverse effects, as their safety profile is not well characterized. Notably, considering that the study is a pre-print, it still needs to be reviewed by other scientists in the field. With that said, if everything holds up, we may see telomere Rivers as a therapeutic option for aging in the future.