Key Points:
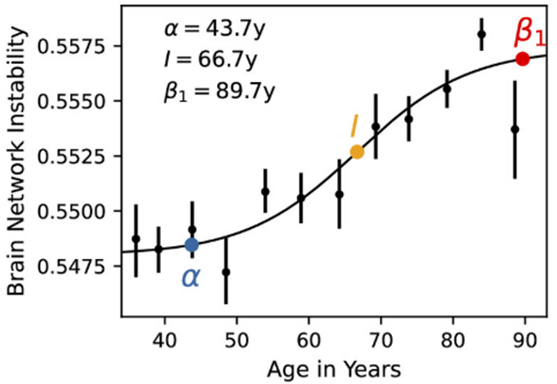
- The researchers’ data suggest that brain aging follows a non-linear trajectory, beginning around age 44, reaching peak acceleration around 67, and plateauing around 90.
- A reduced ability of neurons to use the sugar glucose as fuel with age is correlated with abnormal brain signaling, which has been linked to cognitive decline.
- Supplementing with an alternative fuel besides glucose that neurons can metabolize stabilizes deteriorating brain signaling networks, especially when given during midlife.
A landmark study from Mujica-Parodi and colleagues of the State University of New York at Stony Brook, published in PNAS, suggests that brain aging follows a distinct, non-linear trajectory, beginning in people’s 40s. The researchers also unveiled a correlation between neuronal insulin resistance—where cells begin to lose the ability to use glucose—and the beginning of faulty brain network signaling during people’s 40s. Moreover, they found that supplementing with a cellular fuel source that does not rely on insulin signaling, the ketone 𝛽-hydroxybutyrate, stabilized deteriorating brain network signaling when given during midlife (between ages 40 and 59). These findings suggest that metabolically intervening with ketones during a midlife window could help prevent cognitive aging.
“Understanding exactly when and how brain aging accelerates gives us strategic timepoints for intervention,” said lead author Mujica-Parodi in a press release.“We’ve identified a critical midlife window where the brain begins to experience declining access to energy but before irreversible damage occurs, essentially the ‘bend’ before the ‘break.’ During midlife, neurons are metabolically stressed due to insufficient fuel; they’re struggling, but they’re still viable,” explained Mujica-Parodi. “Therefore, providing an alternative fuel during this critical window can help restore function. However, by later ages, neurons’ prolonged starvation may have triggered a cascade of other physiological effects that make intervention less effective.”
Deteriorating Whole-Brain Inter-Region Signaling As a Marker of Brain Aging
Because research suggests that whole-brain signaling deteriorates with age, Mujica-Parodi and colleagues analyzed the brain’s aging trajectory by measuring declining functional signaling between brain regions. To do so, they used data on 19,300 participants from four large-scale datasets. Contrary to a previously assumed gradual linear decline in brain function, the researchers uncovered an age-related brain network signaling deterioration that followed an S-shaped statistical curve. More specifically, this declining brain region network signaling began around age 44, reached peak acceleration around 67, and plateaued around 90.
Previous work had shown that an age-related loss of energy (impaired metabolism) in neurons impacts signaling between brain regions. However, whether this impaired neuronal metabolism is the cause or result of vascular dysfunction and inflammation remained unclear. Getting to the bottom of what pathological process occurs first in the brain could suggest what dysfunctional process may be the most crucial underlying factor contributing to brain aging.
In their attempt to uncover what abnormal brain processes occur first during aging, the researchers compared markers of age-related metabolic, vascular, and inflammatory changes. In doing so, they found that metabolic changes consistently preceded vascular and inflammatory changes. These findings suggested that underlying metabolic changes occur first in the brain and give rise to processes of aging, like vascular deterioration and inflammation.
Mujica-Parodi and colleagues turned to assessing gene expression levels to pinpoint what specific aspects of brain metabolism may go awry during aging. Their analysis of gene expression levels implicated a neuron-specific glucose transporter called GLUT4 and a lipid transport protein called APOE (known as a risk factor for Alzheimer’s disease) that were associated with these patterns of brain aging. Because GLUT4 and APOE are both insulin-dependent transporters, these findings suggest that neuronal insulin resistance is involved in impaired brain metabolism, which precedes and may give rise to vascular changes and inflammation during aging.
Supplementing with Ketones Stabilizes Brain Signaling During Aging
Moreover, a gene expression analysis also identified a neuronal ketone transporter, MCT2, as a possible protective factor against deteriorating brain signaling. Since the researchers’ data suggest that this ketone transporter may protect against deteriorating brain signaling, Mujica-Parodi and colleagues surmised that the brain may still utilize ketones as fuel instead of glucose during some periods of aging. Thus, they ran an interventional study to test whether enhancing ketone availability in the brain with supplementation of the ketone 𝛽-hydroxybutyrate—an energy source other than glucose that bypasses insulin signaling— improves brain signaling during aging.
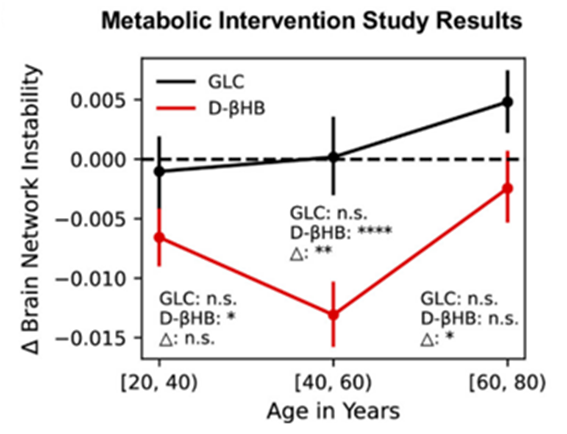
In their interventional study, Mujica-Parodi and colleagues used 101 participants to test differences between consuming a bolus of the ketone 𝛽-hydroxybutyrate or a bolus of glucose on brain signaling. Interestingly, supplementing with 𝛽-hydroxybutyrate ketones conferred stunning effects. In that sense, in younger participants between the ages of 20 and 39, the ketones moderately improved brain signaling. Yet, in middle-aged participants (between the ages of 40 and 59), the researchers found an 84.62% greater brain network stabilization compared to the young group. This midlife effect correlates with a period of accelerated metabolic stress. Comparatively, in older adults aged 60 to 79, when brain signaling deterioration hit maximum acceleration, 𝛽-hydroxybutyrate ketones conferred a much lower impact than the other groups. These findings support Mujica-Parodi and colleagues’ notion that a critical midlife window exists during which metabolic interventions may improve brain function and perhaps even prevent deteriorating function.
A Midlife Critical Window to Counteract Brain Aging
Current treatments targeting brain aging address disease symptoms, such as impaired cognition, often too late for interventions to have meaningful effects. This research from Mujica-Parodi and colleagues suggests that certain metabolic interventions, whether through dietary approaches like fasting to increase circulating ketones or ketone supplementation with 𝛽-hydroxybutyrate, may work against deteriorating brain function when started in people’s 40s. Beginning such a metabolic intervention during midlife would happen well before symptoms of cognitive decline occur.
“This represents a paradigm shift in how we think about brain aging prevention,” said Botond Antal, PhD, the study’s first author. “Rather than waiting for cognitive symptoms, which may not appear until substantial damage has occurred, we can potentially identify people at risk through neurometabolic markers and intervene during this critical window.”
A key limitation of the study is that Mujica-Parodi and colleagues did not test whether supplementing with ketones enhances cognitive function. The researchers could have measured whether ketones improve aspects of memory and problem solving, both facets of cognition. Instead, they showed that ketone supplementation significantly improves inter-brain region signaling, especially during the identified midlife critical window. To get a clearer picture of the potential functional benefits of supplementing with ketones during a possible midlife critical window, future research should explore how 𝛽-hydroxybutyrate supplementation affects critical aspects of cognition. On the other hand, a counterargument against testing ketones’ effects on cognition could be that the participants were healthy adults and that they would not exhibit cognitive decline or have any measurable cognitive deficits.
Finally, for people in their middle-aged years who may wish to find whether ketone supplementation improves their brain function, 𝛽-hydroxybutyrate ketone supplements are available online and in nutrition stores. As suggested in the study, such supplements could have their maximal effects between the ages of 40 and 59.