Key Points
- Nicotinamide enhances the myelination of healthy neurons and drastically mitigates myelin loss (demyelination) upon damage in mouse brain tissue slices.
- Nicotinamide increases myelination at the site of demyelination in a mouse model with demyelination.
- Treatment with nicotinamide suppresses the accumulation of brain cells called microglia and astrocytes that initiate a nervous system inflammatory immune response at the site of demyelination in mice.
Myelin is a layer of fat that wraps around axons — protrusions from neurons that propagate electrical signals in the nervous system, including within the brain. Evidence has shown that myelin loss — demyelination — occurs as we grow older and that it contributes to the pathology of age-related neurological conditions like Alzheimer’s disease. For these reasons, researchers have sought pharmaceutical and nutraceutical remedies to stymie the progression of demyelination as we age.
Published in Frontiers in Cellular Neuroscience, Karagogeos and colleagues from the University of Crete in Greece show that supplementing with the NAD+ precursor nicotinamide enhances myelination with and without exposure to the demyelination-inducing agent lysolecithin (LPC) in mouse brain tissue slices. The researchers go on to show that 400 mg/kg/day injections of nicotinamide improves brain remyelination in LPC-exposed mice. Moreover, nicotinamide suppresses the migration and accumulation of brain cells called microglia and astrocytes — cellular processes called microgliosis and astrogliosis, respectively — that initiate an inflammatory nervous system immune response at the site of LPC-induced damage. Altogether, these findings suggest that nicotinamide promotes myelination and stimulates remyelination after demyelinating events like LPC exposure through suppressing inflammatory microgliosis and astrogliosis responses.
Nicotinamide Promotes Remyelination Against Demyelinating Lesions
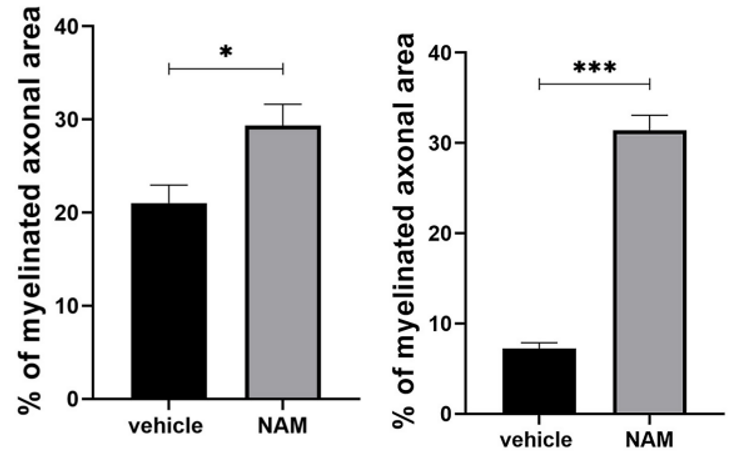
To find whether nicotinamide has any effect on myelination, Karagogeos and colleagues treated mouse brain slices in laboratory dishes with nicotinamide either with or without LPC treatment. Interestingly, even without demyelinating LPC treatment, nicotinamide increased the percentage of myelinated axons. With LPC exposure, the demyelinating agent more than cut myelination in half. However, nicotinamide restored myelination in the LPC-exposed mouse brain slices. These data suggest that nicotinamide treatment promotes the myelination of healthy axons and stimulates remyelination after a demyelinating event like LPC exposure.
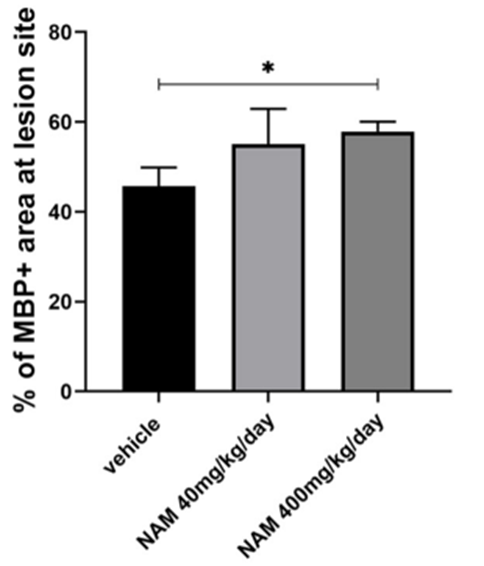
Because nicotinamide enhances normal myelination and promotes remyelination in mouse brain tissue slices, Karagogeos and colleagues sought to find whether the NAD+ precursor has similar effects in living animals, mice specifically. Karagogeos and colleagues examined nicotinamide’s effects on myelination in a brain region with a high number of axons and myelin — the corpus callosum — a structure that fuses the brain’s two hemispheres together. The researchers found that high-dose nicotinamide treatment increased myelin production at the LPC-induced lesion site in the corpus callosum, providing evidence that it stimulates remyelination in mice.
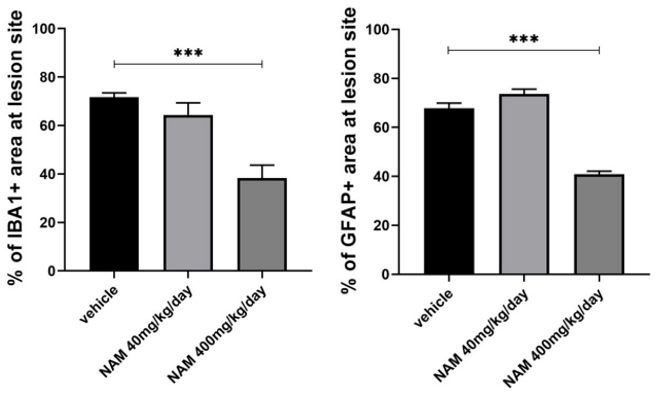
To get a better idea of how nicotinamide triggers remyelination, Karagogeos and colleagues measured the numbers of microglia and astrocytes at the LPC lesion site in the corpus callosum. Elevated microglia and astrocyte numbers in the lesion site would indicate microgliosis and astrogliosis, respectively, where the nervous system mounts an inflammatory immune response. The researchers found that higher nicotinamide doses significantly blunted the microgliosis and astrogliosis responses as seen with significantly lower numbers of focal lesion microglia and astrocytes. These results suggest that nicotinamide promotes remyelination in the brain by reducing microgliosis and astrogliosis.
“Our data support that [nicotinamide] triggers myelin production in the LPC focal demyelination model in vivo by providing a more favorable microenvironment for remyelination,” said Karagorgeos and colleagues.
Whether Supplementing with NAD+ Precursors Remyelinates the Brain During Aging
The study shows that the NAD+ precursor nicotinamide stimulates myelination and even remyelination after exposure to a demyelinating event like LPC exposure. Furthermore, nicotinamide allays the inflammatory cellular environment that goes with a demyelinating-related lesion from microgliosis and astrogliosis. In this way, nicotinamide makes the production of myelin easier for nervous system cells called oligodendrocytes, which synthesize myelin.
Results from the study suggest that supplementing with an NAD+ precursor like nicotinamide or nicotinamide mononucleotide (NMN) can promote myelination. This brings up the question of whether LPC-induced demyelination mimics demyelination from aging and age-related diseases like Alzheimer’s disease and whether these findings apply to aging. Since microgliosis and astrogliosis play active roles in aging and age-related disease brain pathology, it seems likely that supplementing with NAD+ precursors would in fact promote remyelination during aging.