Key Points
- In aged humans, mice, rats, and killifish, longer gene transcripts (mRNA) have lower abundance, while shorter transcripts display higher quantities, comprising unbalanced gene activity.
- Longevity-promoting compounds like rapamycin and resveratrol reverse this age-related gene activity imbalance.
- The longer transcripts primarily encompass longevity-promoting genes, while shorter transcripts are linked to accelerated aging.
Studies examining how gene activity fluctuations over an organism’s lifespan affect aging have focused on the abundance of mRNA transcripts. Yet this method of analysis is limited by not looking at specific features of gene activity that may affect how all genes within cells – the genome – are converted to proteins. This begs the question of what genomic features not examined by looking principally at mRNA abundance may drive aging. By analyzing studies across multiple species, Northwestern University researchers have recently posed the question of whether gene transcript length could contribute to aging.
Published in Nature Aging, Amaral and colleagues from Northwestern University in Illinois found that aging is associated with lower gene activity for longer gene transcripts and higher gene activity for shorter gene transcripts. Their findings applied across multiple species, including humans, mice, rats, and killifish. Interestingly, pro-longevity compounds like resveratrol and rapamycin reversed this unbalanced gene activity. Moreover, the longer gene transcripts with lower activity with age included genes associated with a longer lifespan, while the shorter ones with more activity were associated with shorter lifespans. These findings may help to further our understanding of cellular mechanisms causing gene activity imbalance to pave the way for more precise pharmaceutical therapies that target these mechanisms.
“The changes in the activity of genes are very, very small, and these small changes involve thousands of genes,” said Northwestern’s Thomas Stoeger, the study’s lead author, in a press release. “We found this change was consistent across different tissues and in different animals. We found it almost everywhere. I find it very elegant that a single, relatively concise principle seems to account for nearly all of the changes in activity of genes that happen in animals as they age.”
Unbalanced Gene Activity
To find what genomic features drive aging, Amaral and colleagues used artificial intelligence to analyze what molecular features are associated with age-related gene activity changes in mice. After considering 2,236 molecular features, the Northwestern-based research team uncovered that mRNA transcript length was the most influential. Transcript length had more influence on gene activity profiles during aging than transcription factors – proteins that bind to genes and mediate their activity – and gene length – the length of each gene in DNA. These findings supported that Amaral and colleagues should take a closer look at how transcript length is associated with aging.
To find exactly how transcript length relates to aging, Amaral and colleagues directly compared the transcript lengths from 17 tissues in mice throughout the mouse lifespan. The mice examined were aged 4, 9, 12, 18 and 24 months, the equivalent of 26, 46, 58, 64, and 70 years old for humans, respectively. Amaral and colleagues found that longer gene transcripts were lower in abundance in older age groups (9-, 12-, 18-, and 24-month-old mice) relative to the youngest age group (4-month-old mice). At the same time, shorter gene transcripts exhibited higher abundances. These results suggest that reduced activity for longer gene transcripts and increased activity for shorter ones are associated with aging.
To verify that these findings hold true in other species, Amaral and colleagues gathered gene transcript data from previous studies analyzing other species’ gene activity. They found that this same pattern of unbalanced gene activity applies to mice, rats, killifish, and humans. Interestingly, for humans, more unbalanced gene activity was found in the brain compared to other tissues. These findings suggest that this pattern of gene activity with age applies to multiple species, including humans, and across tissue types.
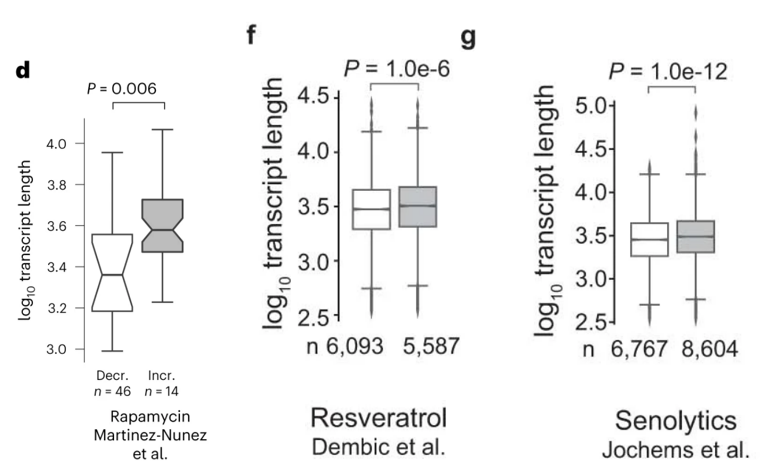
To confirm that the unbalanced gene activity signatures uncovered are linked to aging, Amaral and colleagues compared gene transcript data from mice treated with pro-longevity compounds at different ages. Longevity-promoting compounds, including rapamycin and resveratrol, reversed the unbalanced gene activity signatures, increasing the abundance of longer gene transcripts at older ages. Since longevity-promoting agents reverse the unbalanced gene activity, these data lend further support that unbalanced gene activity is linked to and may drive aging. What’s more, the observation that longevity-promoting compounds may reverse the age-related gene activity imbalance may partially explain their mechanisms of action.
Amaral and colleagues wanted to find how shorter and longer gene transcripts relate to aging. From their data, they looked at genes with the 5% longest and 5% shortest transcript lengths in mice and humans. Their analyses revealed that the shortest gene transcripts contain an excessive amount of anti-longevity-related genes, while the longer transcripts were enriched for pro-longevity genes. These findings support that the declining gene activity associated with longer transcripts and increasing activity for shorter transcripts may significantly drive aging.
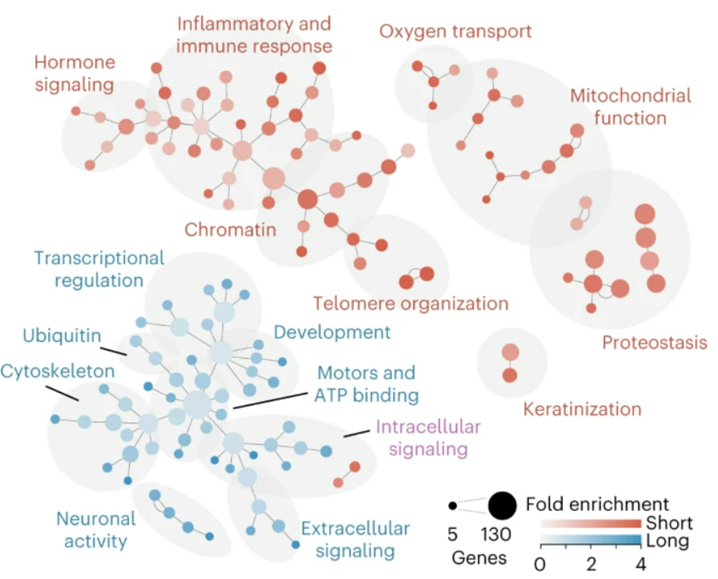
“Some short genes could have a short-term advantage on survival at the expense of ultimate lifespan,” Thomas Stoeger said in the press release. “Thus, outside of a research laboratory, these short genes might help survival under harsh conditions at the expense of shortening the animal’s ultimate lifespan.”
A New Way of Looking at Gene Activation
Research has primarily focused on transcript abundance but not transcript length in an effort to explain diseases and how we age. Maybe we weren’t focused on the right genomic features before. This new understanding of how unbalanced gene activity may drive aging could provide a new way to look at aging mechanisms that leads to better pharmaceutical treatments that may target this genomic feature. Looking at gene activity this way may enable us to see biological phenomena related to aging differently, which may spawn yet-unknown discoveries to counter aging.