Key Points
- Studies have shown eradicating senescent cells with senolytics improves tissue function.
- Researchers propose using senolytics may provide a means to counter age-associated organ deterioration.
- Supplementing mice with senolyics increases their median lifespans by as much as 25%.
As we get older, the number of aged, non-proliferating senescent cells that release a milieu of inflammatory molecules to surrounding cells increases throughout our bodies. The combination of molecules that senescent cells release (the senescence-associated secretory phenotype [SASP]) also induces healthy cells that come into contact with them to become senescent. Although many cells undergo programmed cell death (apoptosis), as we get older, more and more cells become senescent. Since our immunity wanes with age, our immune systems have a harder time disposing of senescent cells, also.
This age-associated accumulation of senescent cells causes tissue inflammation and organ damage. Intriguingly, some researchers propose age-related senescent cell accumulation is one of the reasons we age.
How Senolytics Work and Studies Showing Senolytic Efficacy
Scientists have proposed eliminating senescent cells as a way to counter aging’s adverse effects, namely to reduce inflammation and improve tissue function. For this purpose, researchers have recently developed a new class of pharmaceutical agents called senolytics. The majority of senolytics are derived from a class of molecules called polyphenols, which come from fruits and vegetables. These new drugs can selectively trigger cell death (apoptosis) in senescent cells without affecting the majority of healthy cells.
Senolytics work by targeting pro-survival pathways, which senescent cells use to evade apoptosis. A big challenge to eliminating senescent cells is that there are multiple types of senescent cells that come from different tissues, which rely on different pathways for apoptosis evasion. For this reason, the use of multiple senolytics, each targeting different pathways, may be necessary to terminate large swaths of senescent cells. Moreover, some senescent cells play essential roles in wound healing, so figuring out what each senescent cell population does is critical before deciding which ones to target.
Within the last decade, researchers have begun putting more stock into the idea that getting rid of senescent cells could counter some of aging’s adverse effects. Senescent cells have been found to increase the activation of pro-survival genes for apoptosis resistance. Drugs targeting these pro-survival factors, namely dasatinib and quercetin, selectively kill senescent cells efficiently eliminating senescent cells in different tissue types. The combination of dasatinib and quercetin synergistically provide an enhanced senolytic cell-removing effect by targeting different tissues.
Other studies have shown that removing roughly 30% of senescent cells efficiently slows age-related decline. These studies provided evidence that the selective elimination of senescent cells may be a means to alleviate age-related diseases and promote longevity.
More recent studies have come to light showing that senolytic treatments can improve cardiovascular health. For example, a mouse study showed that dasatinib and quercetin treatments alleviate high cholesterol and vascular calcium buildup, two factors associated with age-related cardiovascular decline. Senolytics have also been proposed to treat type II diabetes and skin aging.
Examples of Senolytics and Clinical Trials
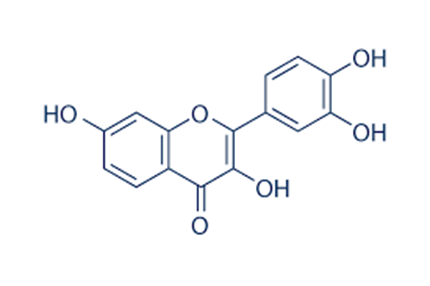
A number of studies have shown the efficacy of the senolytic combination dasatinib and quercetin. Dasatinib is a chemotherapeutic developed by Bristol-Myers Squibb used to treat leukemia. Although it has been shown to effectively eliminate certain types of senescent cells, it comes with multiple side effects, including headaches, difficulty breathing, diarrhea, vomiting, and tiredness. Quercetin, on the other hand, isn’t associated with any adverse side effects and is derived from plants, fruits, and vegetables such as capers, radishes, cilantro, and cranberries.
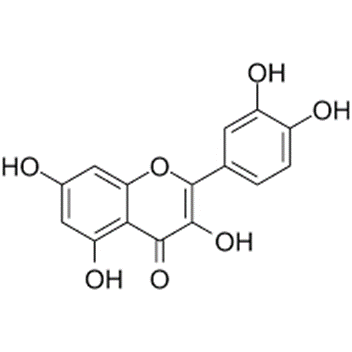
Another molecule found to have senolytic properties is fisetin. Fisetin comes from fruits and vegetables such as strawberries, apples, onions, and cucumbers and has few, if any, side effects. Supplementing mice with fisetin has been shown to increase median lifespan by ~25%. Fisetin has also been shown to have twice the senolytic potency as quercetin.
Senolytic research has been a burgeoning field over the last few years, with many companies developing this drug type. Some senolytics are currently under clinical investigation to find whether they improve age-related health conditions like osteoarthritis, Alzheimer’s disease, and fatty liver disease. Results from these studies will tell us whether aged adults can use senolytics to prevent age-related diseases and increase the number of years they live in overall good health.
While the effects of senescent cell accumulation in the body as we get older need further research, the evidence showing that it plays a key role in aging makes it a target for anti-aging drugs. As such, senolytics present a promising new option to selectively eliminate senescent cells to reduce inflammation and improve tissue health. Using senescent cells as drug targets could be one of the best options we have to slow aging and prevent age-related diseases.