A group of diseases that affect muscle mitochondria – energy-producing “power plants” within cells – are termed mitochondrial myopathies. The manifestation of these diseases can result in fatigue and weakness in eye muscles, and exercise intolerance – a condition called progressive external ophthalmoplegia. Curative treatments have yet to be elucidated for these diseases, however, therapeutics using vitamins provide promising improvements.
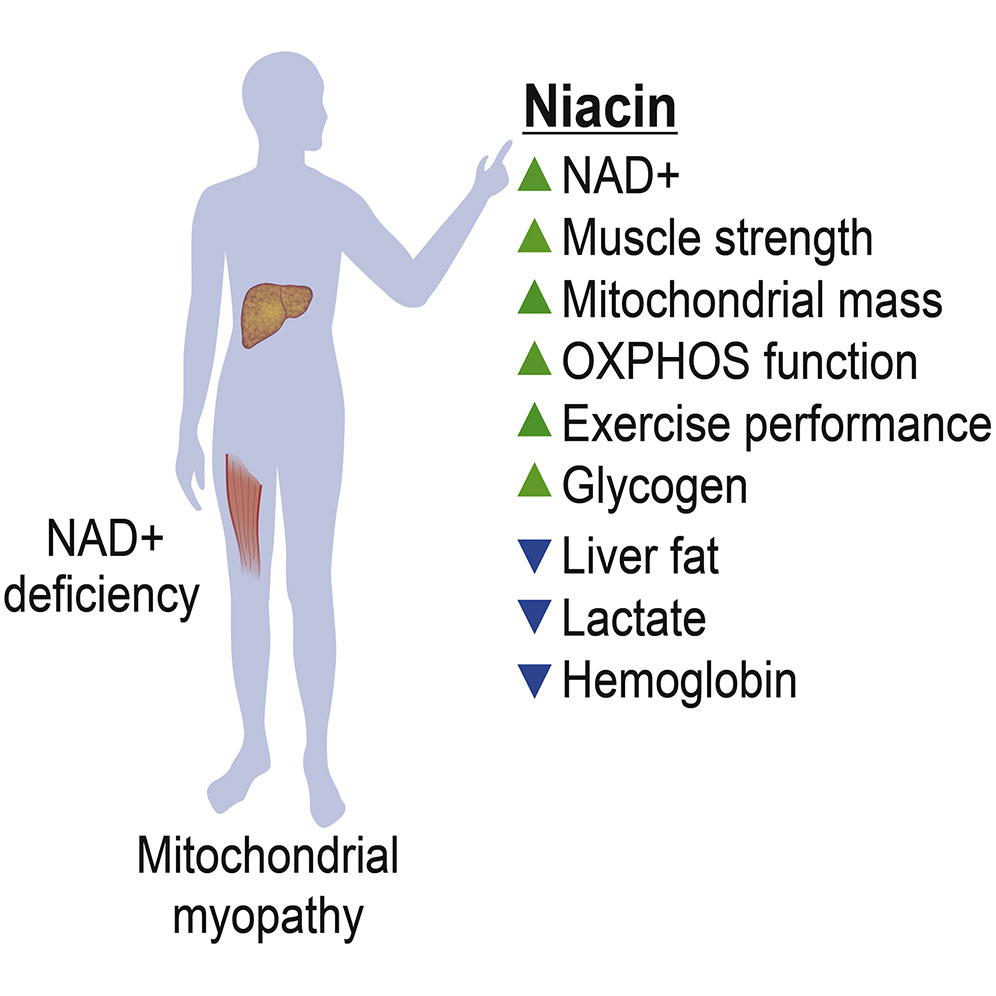
Clinical trial data (NCT03973203) published in Cell Metabolism by Pirinen and colleagues from the University of Helsinki reported that decreased levels of nicotinamide adenine dinucleotide in blood and muscle tissue can result from mitochondrial myopathies. Optimal mitochondrial function relies on sufficient levels of NAD+. Niacin is a precursor to NAD+ and can exist in the form of vitamin B3. The data showed that increased levels of NAD+ in blood and muscle tissue, improved disease signs, and elevated muscle strength and performance in human subjects could be achieved with niacin treatment.
Can vitamin B3 forms alleviate mitochondrial myopathy symptoms?
Metabolic regulation, longevity, aging, and disease are all affected by NAD+. This pivotal metabolite has been investigated extensively in model organisms, and aging along with the development of degenerative diseases has been linked to the depletion of NAD+ in rodents. However, it is still unknown if patients with degenerative disorders exhibit NAD+ depletion or improvement of symptoms through NAD+ repletion.
Interestingly, previous investigators used a mouse model for mitochondrial myopathy that showed supplementation with nicotinamide riboside, an NAD+ precursor vitamin B3, increased mitochondrial biogenesis and thus disease symptoms were prevented and delayed. Vitamin B3 exists in the following three forms: nicotinamide, niacin, and nicotinamide riboside. Animal studies have indicated vitamin B3’s ability to increase intracellular NAD+ levels, which then leads to improved function of diseased mitochondria.
“Our main question was whether NAD+ levels are depleted in mitochondrial dysfunction, as mitochondria are regulating NAD+ concentrations, and if so, whether NAD+ deficiency can be restored in the tissues of the patients,” said the investigators. So, niacin was used by Pirinen and colleagues to test its ability to recover dysfunctional mitochondria improve symptoms of mitochondrial myopathy. Furthermore, niacin was the chosen form of vitamin B3 because it was shown to be safe and effective at high doses to treat patients with hypercholesterolemia.
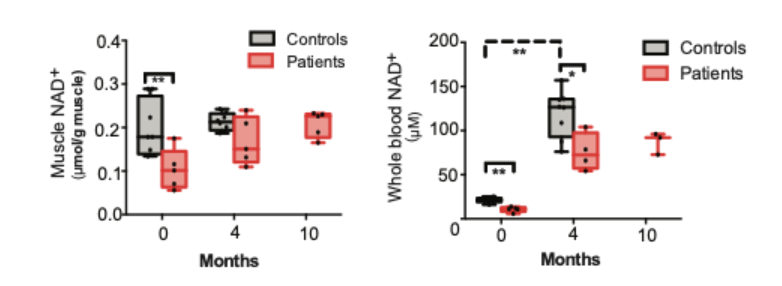
Niacin repletes NAD+ deficiency in human muscle and blood
Niacin was slowly supplemented by the research team to PEO patients along with a healthy control group for four months. The initial administered dosage was 250 mg/day, and this was gradually increased to 750 or 1000 mg/day during the four-month period. Observations of the treatment effects on patients were then monitored up to 10 months. Pirinen and colleagues demonstrated that NAD+ deficiency was linked to adult-onset mitochondrial muscle disease, and this phenomenon is known as myopathy-induced vitamin B3 deficiency. All subjects exhibited up to an 8-fold increase in blood NAD+, and the concentration of NAD+ in the muscles of patients the level of their controls.
In addition, data presented niacin’s ability to rescue NAD+ levels, and restore muscle and systemic NAD+. While there was a 5-fold increase in blood NAD+ in healthy patients, NAD+ in muscles was not increased through niacin supplementation. “Our data implicate the potent effects of vitamin B3 forms on metabolism and present blood NAD+ analysis as a powerful tool to identify patients and individuals with NAD deficiency,” said the investigators.
Myocardial myopathy patients show enhancement of mitochondria production and muscle strength through niacin supplementation
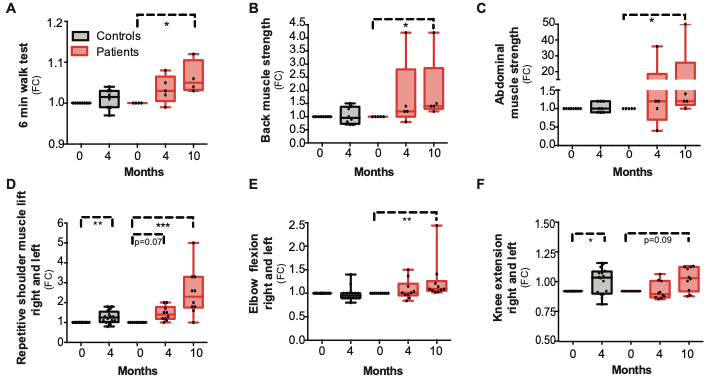
Similar responses in the metabolism of patients occurred through niacin supplementation. All subjects displayed increases in mitochondrial generation and muscle strength; however, anemic tendencies were seen in some patients. The muscle metabolite profiles moved toward controls in subjects, while liver fat went down by 50%.
The remarkable observed metabolic effects after niacin supplementation probed Pirenen and colleagues to investigate how the performance of patients was affected by niacin. Myocardial myopathy patients exhibited improved muscle strength after niacin was supplemented for 10 months; however, data showed a variation in improvement for different muscle groups. On average, the following fold changes were seen for varying muscle groups: 2-fold in back muscles, 10-fold in abdominal muscles, and 2.5-fold in upper extremities (strength of elbow and shoulder flexion). Furthermore, only slight improvements were seen in the six-minute walking test in addition to lower extremities (1.1-fold in knee extension strength).
“We demonstrate that niacin remarkably restores muscle and systemic NAD+ and provides metabolic and functional benefits for patients with mitochondrial myopathy, indicating that NAD+ deficiency contributes to disease progression,” said the investigators in the article.
Pirinen and colleagues emphasize how metabolism is greatly influenced by vitamin B3. This study demonstrates how the progression of mitochondrial myopathy can result from NAD+ deficiency, and it also suggests that identification of NAD+ deficiency could be conducted through a blood NAD+ test. The researchers postulate that the data collected suggests that PEO patients could benefit from niacin therapy.
Will niacin be used as a treatment for mitochondrial myopathy in the future?
“Our pilot study is a proof-of-principle of niacin effects on mitochondrial myopathy,” said Pirinen and colleagues in their article. The determination of the ability to assist delaying disease progression through early niacin treatment immediately after diagnosis still requires further investigation. Furthermore, the efficacy of niacin supplementation in this study was based on its effect on patients with hypercholesterolemia, and thus probes for further analysis to fully elucidate the optimal dose for mitochondrial myopathy.