Key Points:
- Sleep deprivation leads to alterations in mitochondrial health and a protein complex known as DREAM.
- Harmine, which blocks DREAM, mitigates the cellular aging triggered by sleep deprivation.
- Blocking DREAM allows cellular repair genes, such as antioxidant genes, to be activated.
Inadequate sleep increases the risk of type 2 diabetes, heart disease, cancer, Alzheimer’s disease, and early death. As such, adequate sleep is fundamental to healthy aging. However, many factors may prevent adequate sleep, from societal pressures to a snoring spouse.
In addressing the consequences of inadequate sleep, which is now considered a serious public health concern, scientists have begun to find ways of alleviating the cellular aging it triggers. In a new study (not yet peer-reviewed), scientists from the Leibniz Institute on Aging in Germany may have discovered a compound that can counteract the ravages of poor sleep.
Sleep Deprivation Alters DREAM and Mitochondrial Health
By analyzing the brain tissue of mice, the Leibniz researchers found that certain genes were elevated during wakefulness but not during sleep. Specifically, they found that parts of the DREAM complex were elevated during wakefulness but not during sleep. The DREAM complex, a master regulator of cell cycle progression and DNA repair, lies at the heart of the study. Importantly, sleep deprivation altered the dynamics of DREAM, suggesting it could play a role in the adverse health consequences of poor sleep.
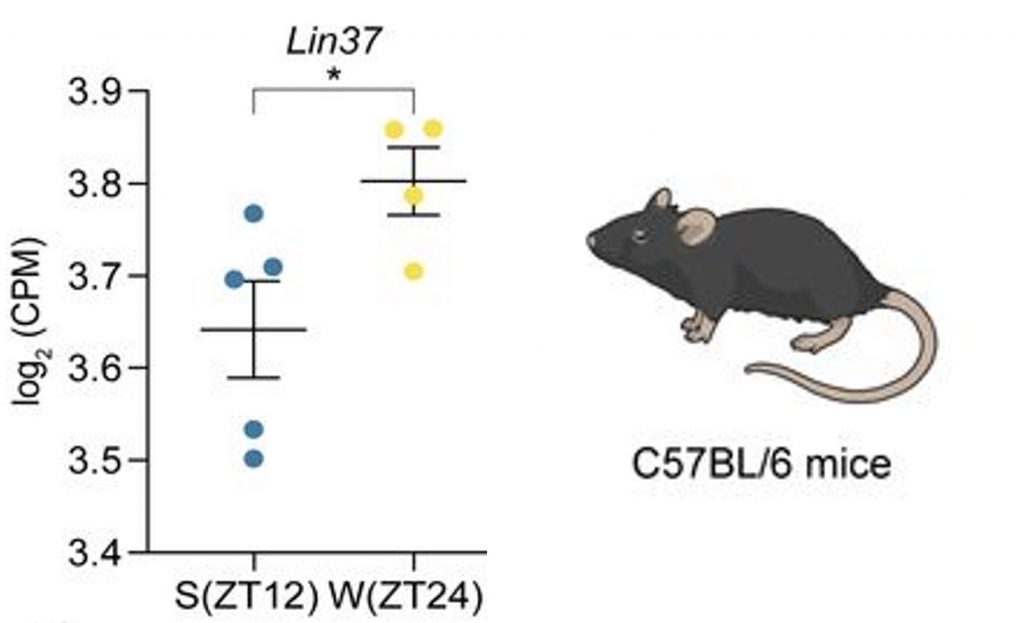
The researchers also found that sleep deprivation induced changes in genes associated with fundamental cellular drivers of aging, including mitochondrial health. Mitochondria are the primary producers of cellular energy. Studies have shown that mitochondrial activity is ramped down during sleep to allow for mitochondrial repair and restoration. However, the researchers found that genes related to mitochondrial function were not ramped down in sleep-deprived mice, which could lead to mitochondrial dysfunction and accelerated aging.
Harmine Counteracts Age-Accelerating Consequences of Sleep Deprivation
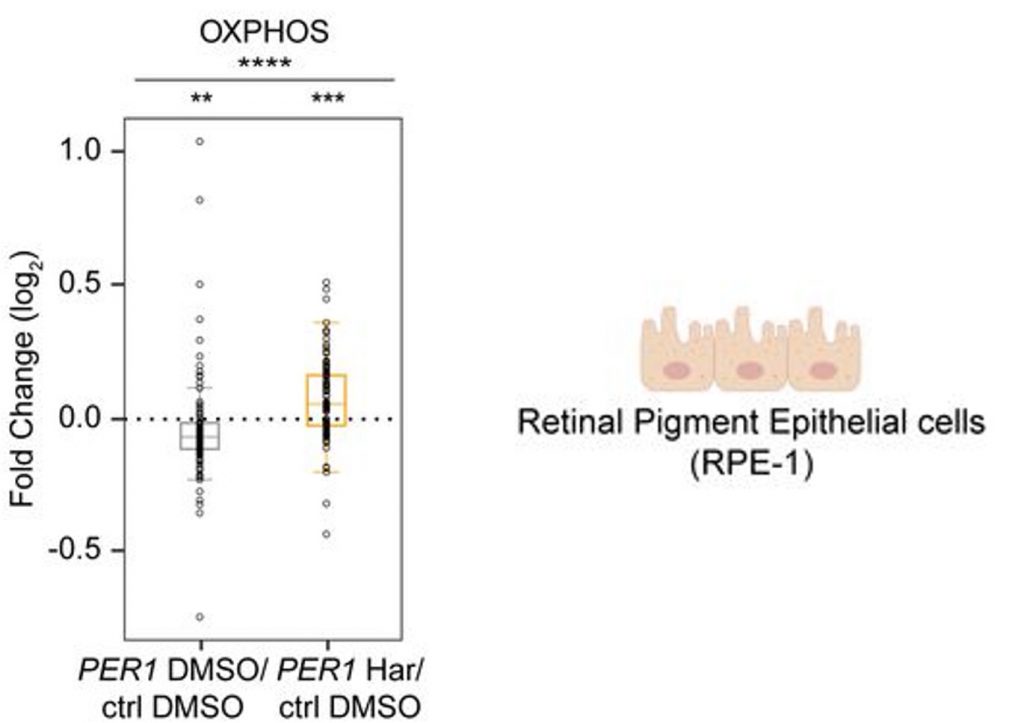
Our natural sleep-wake cycle, which repeats about every 24 hours — our circadian rhythms — plays a critical role in sleep optimization. To model sleep deprivation, the researchers deleted a gene important for circadian rhythms in human retinal cells. In response to the circadian rhythm disruption, the researchers found that genes related to mitochondrial function were dampened. However, this dampening was prevented if the DREAM complex was blocked by the drug Harmine.
The researchers performed a similar experiment in C. elegans worms, whereby they genetically disrupted the circadian rhythms of these microscopic animals to model sleep deprivation. It was shown that the “sleep-deprived” worms had motility defects, an indicator of worsened muscle function, which occurs in old humans. However, Harmine was shown to prevent these motility defects.
Together, these preliminary experiments in human cells and worms suggest that Harmine can potentially counteract the harmful consequences of sleep deprivation by blocking the DREAM complex. The results suggest that it may potentially do so by improving mitochondrial health, which tends to decline with sleep deprivation.
Harmine May Prevent Sleep Deprivation-Induced DNA Damage
Based on their results from experiments done in worms, mice, and human cells, the Leibniz researchers developed a model for how Harmine could potentially counteract the cellular aging induced by sleep deprivation.
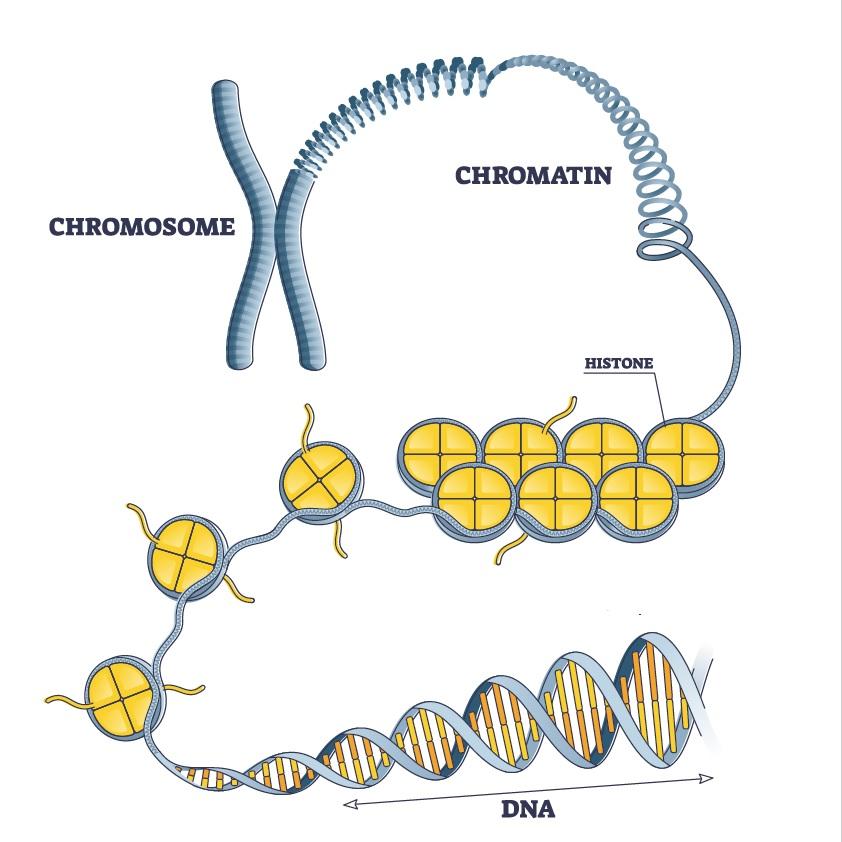
Histones and Chromatin
To fit into the nucleus of a cell, our DNA is wrapped around proteins called histones and coiled into larger structures called chromatin. For genes to be turned on, the DNA for those genes must be exposed to the transcriptional machinery of the cell. For this to occur, the chromatin must uncoil, and the DNA containing the genes must be unwrapped from its histone.
A key finding of the Leibniz researchers was that during wakefulness, histone levels were elevated, along with the DREAM complex, in worms. The increase in histones suggests that chromatin is more coiled, or compacted, during waking hours. Moreover, the findings suggest that more DNA is wrapped around histones.
The DREAM Complex Model
If it were to be applied to humans, the model posits that when we are awake, the DREAM complex is elevated, leading to higher chromatin compaction. While compacted into chromatin, DNA is shielded from cellular stressors. For example, oxidative stress, which is thought to contribute largely to cellular aging, can damage DNA and shorten telomeres. Chromatin compaction may protect against DNA damage and telomere shortening.
Moreover, without being exposed to the DNA repair machinery of the cell, DNA repair is reduced during chromatin compaction and wakefulness. At the same time, the genes turned off by chromatin compaction are involved in cellular repair mechanisms, including DNA repair. Thus, while we are sleeping, the DREAM complex decreases along with chromatin compaction, allowing for cellular repair genes to be turned on. This includes genes involved in mitochondrial health and antioxidant genes, which counteract oxidative stress.
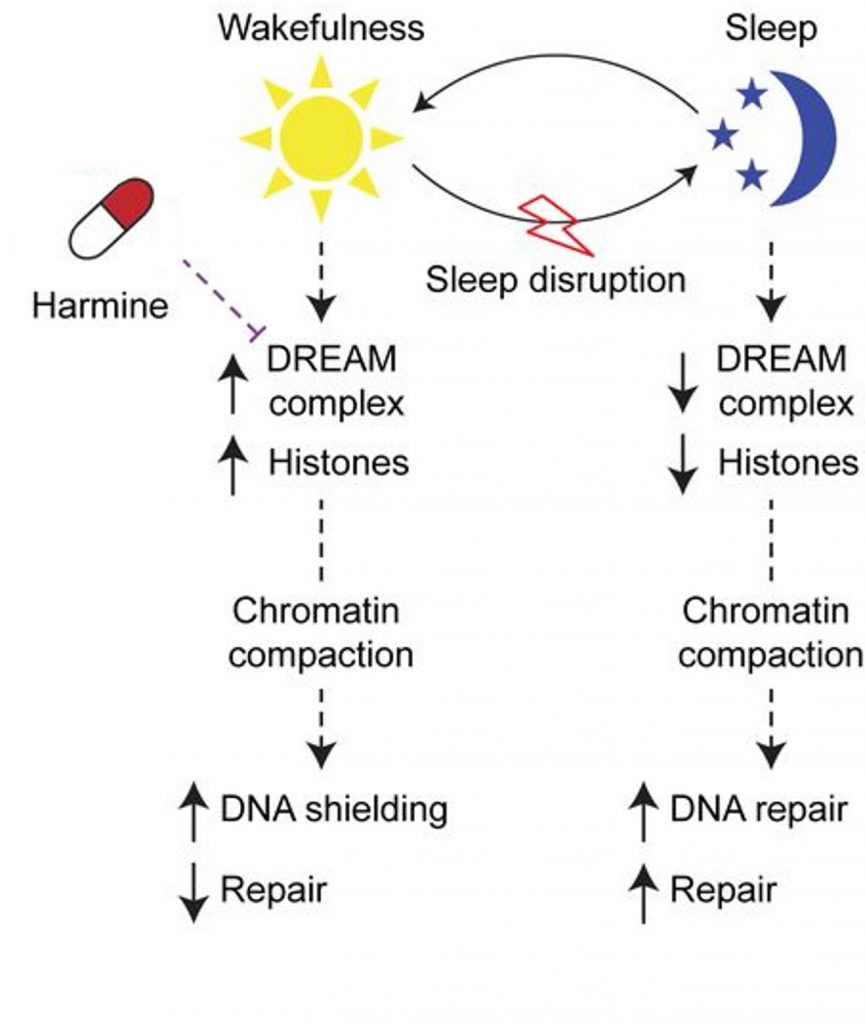
When we are sleep deprived, the DREAM complex remains elevated, and genes responsible for damage repair, including antioxidant genes, remain turned off. This would inevitably lead to cellular damage and accelerated aging. However, by blocking the elevation of DREAM, Harmine reverses chromatin compaction, allowing for cellular repair genes to be turned on. In this way, Harmine could prevent the cellular aging induced by sleep deprivation, according to this model.
Alternatives to Harmine
Considering the widespread issue of sleep deprivation in modern society, the implications of this discovery are profound. If DREAM remains highly active due to insufficient sleep or disrupted circadian rhythms, our cells are effectively denied their crucial window for repair. This prolonged suppression can lead to an accumulation of cellular damage, contributing to aging and age-related diseases.
However, Harmine may be difficult to come by, and this research is still in its early stages; much more work is needed before any such therapeutic applications can be realized. In the meantime, there may be other options for preventing the age-accelerating effects of sleep deprivation. For example, Sergagon Biosciences recently launched Enlivien, designed to target the cellular consequences of sleep deprivation, such as oxidative stress and mitochondrial health.