Key Points:
- In the first protein aging atlas, researchers found that organs age at different rates and that vascular aging is central to body aging.
- Their massive dataset on protein aging was used to create aging clocks that estimate organ aging rates.
- Around 50, aging accelerates, increasing the risk of age-related diseases like type 2 diabetes.
We all know that our bodies change as we get older, but until now, scientists did not know exactly how this happens at the molecular level in different organs. A landmark new study might change that.
Scientists from the Chinese Academy of Sciences have made the first-ever atlas of how proteins change in different human tissues over five decades of life. This gives us a new way to look at the biology of aging: the proteins that keep our bodies running. Their findings could help us figure out why getting older makes us more likely to get sick and suggest new ways to find and treat age-related decline.
The study, published in the journal Cell, examined over 500 samples from 13 distinct tissues, such as the heart, lungs, skin, muscles, and blood, collected from individuals aged 14 to 68. It showed when and where aging starts, and it also showed that some proteins may actually cause aging, not just reflect it.
Proteins: The Body’s Workhorses
Proteins are crucial for nearly everything the body does. They make up 15–20% of our body weight and are found in every cell. They form the structure of muscles, skin, and bones and carry out essential tasks like repairing tissue, producing hormones, and fighting infection.
But proteins don’t stay constant throughout life. They’re constantly being made, broken down, and replaced. This process—called proteostasis—is tightly regulated, and when it goes wrong, it can lead to serious problems.
The new study shows that as we get older, proteostasis decreases in almost all organs. With the protein recycling process slowed down, damaged proteins build up.
Key Finding #1: Aging Is Not Uniform Across the Body
One of the most striking findings was that different organs age at different rates. All tissues age, but some start earlier.
Even young adults showed aging-related protein changes in the aorta, the body’s largest blood vessel. These early blood vessel changes were linked to inflammation and tissue damage, suggesting that vascular aging may be central to body aging.
The data suggest that blood vessels are an “aging hub” that affects other organs. This could explain why cardiovascular disease increases with age and why maintaining a healthy circulatory system may slow aging elsewhere.
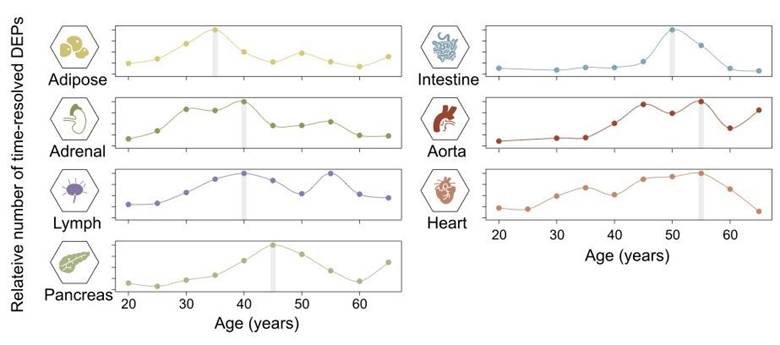
Key Finding #2: Between 35 and 55 Is When Most Organs Undergo Protein Aging
Starting in our mid-40s to mid-50s, many organs in the body go through major changes in the proteins they produce, which can affect how they work. The aorta—the body’s main artery—shows the strongest and most consistent shifts, making it especially sensitive to aging. Even by age 30, some organs, like the adrenal gland, spleen, and aorta, already show early signs of change, hinting that hormone regulation problems may kickstart the aging process. Later changes, around ages 45–55, also involve the pancreas and spleen, which may reflect declines in metabolism and immune function. Overall, these findings suggest that aging doesn’t happen uniformly but affects organs differently, with blood vessels among the first to show wear.
This acceleration of aging increases the risk of chronic diseases like heart disease, type 2 diabetes, and cognitive decline. Understanding that many organs age faster around midlife may help doctors target midlife for early detection and prevention.
Key Finding #3: Blood Proteins Reflect Organ Aging
The researchers also examined blood and tissue proteins. They discovered that many blood protein changes reflect organ function, so blood tests could estimate organ biological ages, a major advance in personalized medicine.
The researchers identified specific proteins in the blood, called “senoproteins,” that increase with age and are associated with inflammation and tissue damage. One such protein, GAS6, stood out for its strong link to both vascular aging and overall aging across the body.
These proteins could serve as biomarkers—molecules that indicate the health or biological age of specific tissues—and may even become targets for future treatments.
Key Finding #4: Proteins Can Drive, Not Just Reflect, Aging
It’s tempting to think aging “happens” to the body, but this study shows that some proteins actively contribute to the aging process. For example, several immune proteins, including complement proteins and immunoglobulins, were found to increase with age and promote inflammation. This chronic, low-level inflammation—sometimes called “inflammaging”—has been linked to many age-related diseases, from arthritis to heart disease to dementia.
The research also uncovered signs of “transcriptome-proteome decoupling”—a breakdown in how well the body follows its genetic instructions to make proteins. In younger people, the genes that get turned on typically produce the corresponding proteins. But as we age, that relationship weakens, leading to mismatches that may further disrupt cell function.
A New Tool: Organ-Specific Aging Clocks
Using their massive dataset, researchers created “proteomic aging clocks” for each organ. These tools use an organ’s protein profile to estimate its age, potentially helping doctors detect aging and identify organ-specific diseases. For example, someone might be 55 years old chronologically but have a liver with the protein profile of a typical 70-year-old. That could trigger further testing or lifestyle changes before symptoms arise.
What This Means for the Future
This research could lay the foundation for a new generation of age-targeted diagnostics and therapies. Here are a few possibilities:
- Blood tests that estimate the biological age of your organs.
- Drugs that block or remove harmful aging-associated proteins.
- Vaccines or immune-based therapies that clear out senescent (aging) cells.
- Early intervention strategies aimed at preventing diseases before they start.
While more research is needed—especially in larger and more diverse populations—the study provides proof of concept for using proteins to measure and potentially influence how we age.
What You Can Do Now
Although most of the tools mentioned above are still in development, the findings reinforce several well-established steps that can help slow aging:
- Protect your heart and blood vessels. Cardiovascular health may influence aging throughout the body. Eat a heart-healthy diet, exercise regularly, and avoid smoking.
- Eat anti-inflammatory foods: Diets rich in fruits, vegetables, whole grains, and omega-3s can help, along with regular physical activity and stress management.
- Reduce inflammatory food intake: Foods known to cause inflammation, according to Johns Hopkins Medicine, and should be avoided included processed meats (e.g., hot dogs, lunch meat), commercial baked goods (e.g., cookies, brownies), deep-fried items (e.g., french fries), sugar-sweetened beverages, or food rich in trans fats (e.g., microwave popcorn, nondairy creamer)
- Stay informed. As new tests and treatments based on aging biology become available, being proactive with your healthcare can help you take advantage of them.
Aging is one of the greatest risk factors for nearly every major disease. But it is no longer a mysterious process that happens in the background. Thanks to studies like this, we are beginning to understand the molecular changes that drive aging—and how we might intervene.
Proteins, once thought of mainly as the products of aging, may actually be its main architects. By learning to read and rewrite their messages, we could usher in a future where aging is not just managed, but truly understood.