Key Points:
- Identifying the causes of aging remains a critical challenge.
- If we could pinpoint such causes of aging, the aging research field could begin targeting them as a means to extend our lives.
- Several current technological advancements, like NAD+ precursors, can counteract these processes.
The aging research field has produced a number of new, groundbreaking anti-aging technologies over the last few years. Among these, nicotinamide adenine dinucleotide (NAD+) precursors, such as NMN and NR, which counteract falling NAD+ levels during aging, can be used to combat possible causative factors of aging. Reviewing possible causative factors contributing to how we grow older and how NAD+ precursors address them is essential for understanding how the supplements work to rejuvenate the body as we age.
Among the factors that may drive aging are the accumulation of DNA damage, the shortening of protective DNA sequences at chromosome ends (telomeres), and altered DNA molecular tagging patterns (methylation). In a publication in Nature Genetics, João Pedro de Magalhães from the University of Birmingham in the United Kingdom describes evidence for these possible causal factors’ influences on aging. Essentially, all of these possible aging contributors may drive our physiological decline with time to some degree and may operate in tandem.
The denominator here is that all of these potential drivers of aging pertain to alterations in DNA. Other theories of aging exist that do not relate to DNA changes, but the DNA-related changes appear to be the most compelling and may hold the key to figuring out how we age and how to influence the process. Along the lines of looking at DNA and the genes it codes for, in the words of de Magalhães,
“I argue that the path forward to assess causality in the field of aging should involve genetic manipulations or associations that allow genes modulating specific aging-related pathways or processes to be empirically assessed for their role in aging.”
Age-Related DNA Damage as a Contributor to Aging
Cells can replace any of their damaged components, except for our inherited genetic code stored in our genome. Our genome is composed of DNA that effectively acts as the blueprint for all of the proteins needed for cellular structures, enzymes, hormones, etc. The deterioration of our genome, caused by the slow accumulation of damage over time, leads to an incomplete blueprint and altered cellular functions. As such, a prevailing theory of aging states that DNA damage accumulation with passing time causes aging.
In support of this theory of aging, several studies have shown that disrupting our body’s natural DNA repair responses accelerates aging and shortens lifespan in mice. However, exceptions to these findings have created doubt regarding the degree to which DNA damage causes aging.
For example, certain strains of mice with increased DNA damage do not age faster than normal, even though they may exhibit a higher incidence of cancer. In this regard, de Magalhães says that it is possible that certain forms of DNA damage, like DNA double-stranded breaks (a complete breakage to the DNA double-helix), contribute to aging more than other forms — like breaks to single strands of DNA. [1] All the same, de Magalhães concludes that there is a lack of conclusive evidence showing DNA damage causes aging.

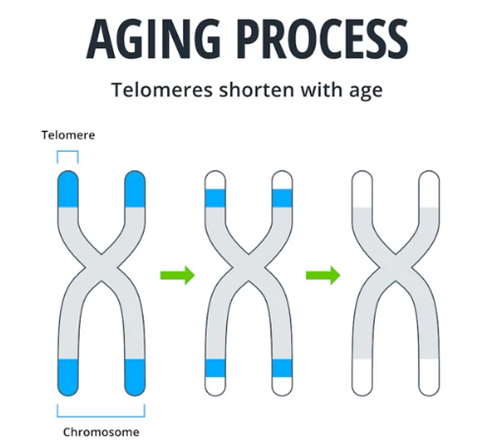
Telomere Shortening May Drive Aging
Our cells store DNA in long strands called chromosomes. Chromosomes have built-in protection for the DNA they store in the form of telomeres. Telomeres are protective caps at the ends of chromosomes. Like the plastic tips at the ends of shoelaces, telomeres protect the important genetic information in our DNA from being damaged during cell division. Every time a cell divides, its telomeres get a little shorter. Eventually, when telomeres become too short, the DNA itself begins to receive damage and the cell can no longer divide properly.
About 20 years ago, the idea that telomere shortening could serve as a causal factor for aging became popular. At that time, researchers discovered that increasing levels of an enzyme that elongates telomeres (telomerase) prevents cells from entering an age-related dysfunctional state called senescence. Senescent cells are thought to be a key contributor to aging, so preventing their buildup with telomerase was proposed as a means to slow aging.
Studies examining the role of elongating telomeres with this enzyme have been moderately supportive of telomere shortening’s role in aging. For example, increasing this telomere-elongating enzyme with gene therapy in mice has been shown to modestly extend lifespan by 13-23%. Along those lines, telomere shortening remains an underexplored possible contributor to aging, and its overall impact on aging, in species besides mice, needs more research.
Do Age-Related DNA Molecular Tagging Alterations Cause Aging or Are They Byproducts of Aging?
Researchers have identified age-related changes to patterns of DNA molecular tags — methylation — to predict people’s ages and chances of dying (mortality). The ways that these methylation-based aging clocks predict aging and mortality remain poorly understood. Moreover, direct manipulation of DNA methylation patterns to determine if they causally influence an organism’s age has not been conducted.
However, partial cellular reprogramming, by increasing the activation of four genes called Yamanaka factors, has been shown to reset methylation patterns and rejuvenate cells. Whether the resetting of methylation patterns causes cellular rejuvenation or whether other factors related to Yamanka factors drive cellular rejuvenation remains to be determined. Thus, whether aging-related alterations to DNA methylation patterns drive aging and whether resetting them with partial cellular reprogramming reverses aging is still unclear.
Combining Compounds to Counteract Multiple Potential Causes of Aging
It may be the case that DNA damage accumulation, telomere shortening, and age-related DNA methylation alterations drive our physiological decline with passing years to varying degrees. If that is true, then finding ways to target all of these potential contributors to aging with a single supplement would be ideal for longevity extension.
Along those lines, the essential molecule nicotinamide adenine dinucleotide (NAD+) is necessary for the function of the DNA repair enzyme, PARP-1. As we get older, the NAD+ levels in our body begin to decline and this is believed to contribute to the increase in DNA damage accumulated when we get older. By increasing NAD+ with NAD+ precursors like nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN), researchers have demonstrated that PARP-1-related DNA repair can be improved. In this way, NAD+ precursors may counteract DNA damage accumulation during aging.
Moreover, another study has shown that boosting NAD+ with precursors increases telomere length in humans. Along those lines, NAD+ precursors may also help to thwart age-related telomere shortening.
Furthermore, Harvard’s David Sinclair has shown that certain compounds partially reprogram and rejuvenate human cells. As such, future pharmaceuticals may be able to combine NAD+ precursors with compounds that partially reprogram cells in the body. Such compounds could address the known potential contributors to aging like DNA damage accumulation, shortened telomeres, and altered DNA methylation patterns.