Key Points:
- NR improves brown fat NAD+ and mitochondrial health.
- NR primes fat for healthy aging by reducing visceral fat and increasing brown fat.
- NR increases the propensity of brown fat to burn calories as heat.
Most of us are familiar with white fat tissue, the fat that stores excess calories and makes us expand. Yet, few may be aware of brown fat tissue, the fat that turns calories into heat. With age, brown fat decreases, contributing to metabolic impairments and energy imbalances that may lead to a shortened lifespan. It follows that preserving brown fat tissue can potentially foster longevity (living longer) by staving off the physical deterioration associated with aging.
As published in the Journal of Physiology, researchers from the University of Campinas in Brazil, led by Dr. Eduardo R. Ropelle, have found that the NAD+ (nicotinamide adenine dinucleotide) precursor NR preserves brown fat tissue in obese mice. In brown fat, they found that NR helps retain healthy mitochondria, a beneficial effect known to support health and longevity when tested in other tissues. These findings add to the growing catalog of studies detailing the beneficial effects of NAD+ precursors on mitochondrial health and disease-free aging.
NR Improves Brown Fat NAD+ Levels and Mitochondrial Health
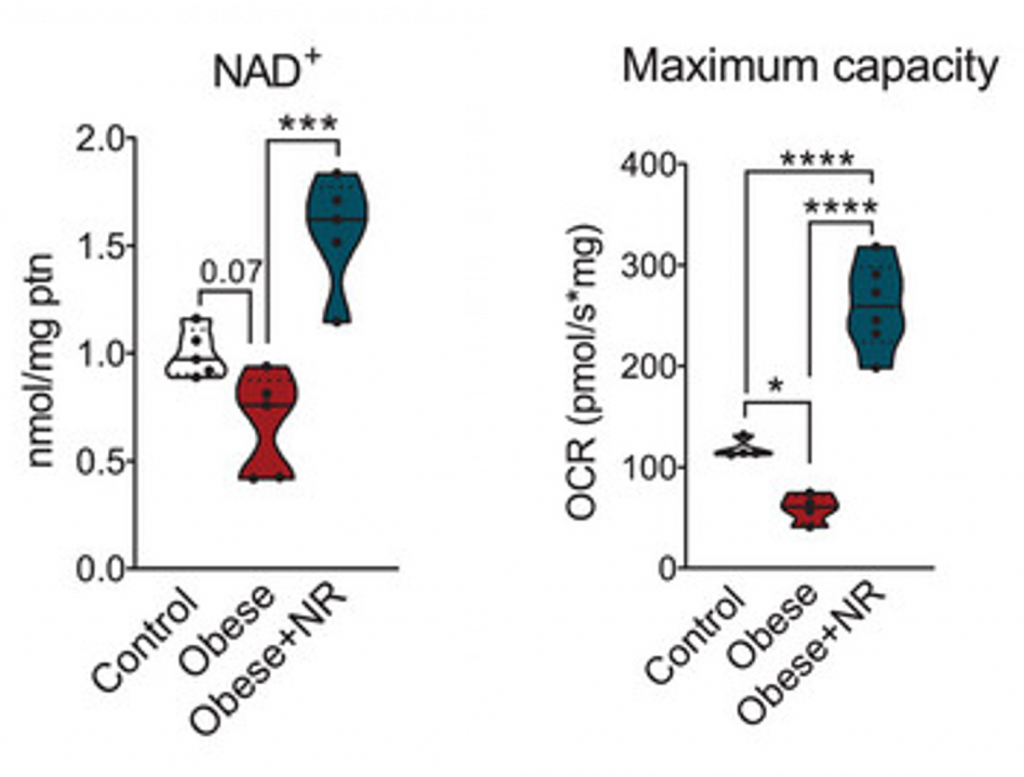
Scientists have proclaimed that “reduced NAD+ levels cause aging and aging-associated disease.” Supplementation of NAD+ precursors like NR elevates NAD+ in multiple tissues, mitigating cardiovascular disease, neurodegeneration, and other age-related diseases. Adding to this list, Dr. Ropelle and his team found that NR elevates NAD+ levels in the brown fat tissue of obese mice. Similar to aging, brown fat tissue is decreased in obese individuals, which is one of the reasons why obese mice were examined.
The researchers also found that NR supplementation maintained brown fat mitochondrial function, as indicated by factors such as the increased capacity to generate cellular energy (ATP). Mitochondrial dysfunction is one of the hallmarks of aging, meaning it is associated with nearly every age-related disease, including cardiovascular disease, neurodegeneration, and metabolic syndrome. Ropelle and team’s findings demonstrate that elevating NAD+ levels in the brown fat of obese mice counteracts mitochondrial dysfunction, implying that similar effects could be achieved in aged mice.
NR Primes Fat for Healthy Aging
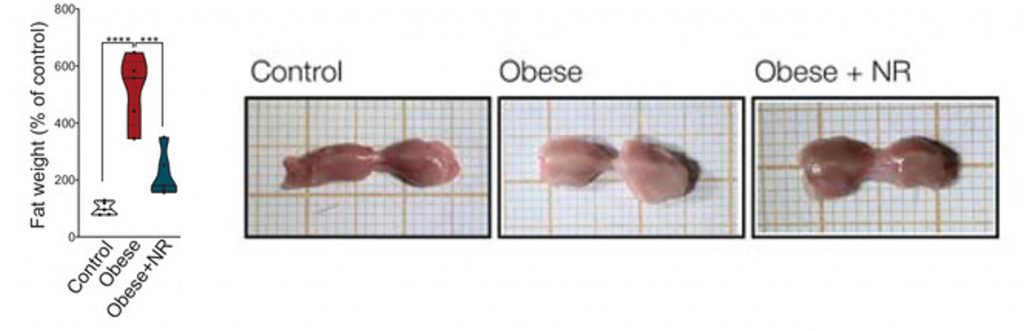
Many animal studies and at least one human study have shown that NAD+ precursors counteract weight gain and obesity. Ropelle and team received similar results, additionally showing that NR greatly reduces visceral white fat, the fat in the abdomen that surrounds the organs and increases dramatically with age. Along with reducing white fat mass, the researchers showed that NR reverses obesity-induced whitened brown fat tissue. This simultaneous reduction in white fat and increase in brown fat could alleviate metabolic impairments associated with obesity and aging.
NR Improves Uncoupling: The Secret to Metabolic Health and Longevity?
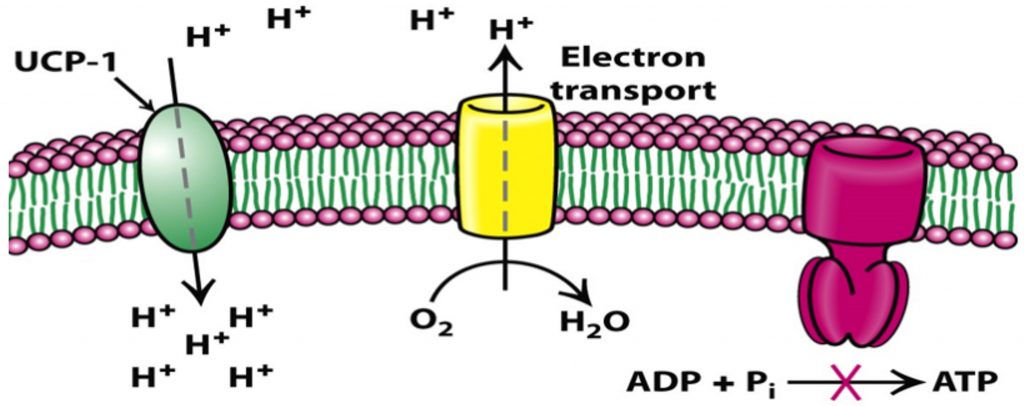
Brown fat is what helps keep us warm, at least as infants. Infants do not shiver, which is another way our body increases temperature in response to cold. To help keep us warm and produce heat, brown fat cells are equipped with a protein called uncoupling protein-1 (UCP-1), found in mitochondria.
How Uncoupling Works
The food we eat is broken down into macronutrients, such as carbohydrates and fats, which are then metabolized to produce electrons that are captured by NAD+. NAD+ transfers electrons to the electron transport chain (ETC) in mitochondria, which causes protons to accumulate inside the mitochondria. Once enough protons accumulate and a gradient forms, the protons pass through a structure called ATP-synthase, which synthesizes ATP.
However, in brown fat cells, instead of passing through ATP-synthase, the protons primarily pass through UCP-1. UCP-1 does not synthesize ATP, but dissipates energy as heat. In this way, UCP-1 uncouples the proton gradient from ATP production. Since excess ATP leads to fat storage, uncoupling calorie intake from ATP production can lead to weight loss. However, in the 1930s, a weight-loss drug targeting this uncoupling process called DNP (2,4-dinitrophenol) was taken off the market for dangerous side effects such as hyperthermia and death.
NR Increases Uncoupling
Despite people overdosing on it in the 1930s, scientists have tested the longevity effects of low-dose DNP on mice, finding that it improves metabolic health and prolongs lifespan. Moreover, studies have shown that UCP-1 declines with age. Based on these findings, it is fair to say that increasing the uncoupling process via elevating UCP-1 levels can potentially reap longevity effects. It may do so by counteracting obesity, which shortens lifespan and contributes to the acceleration of biological aging.
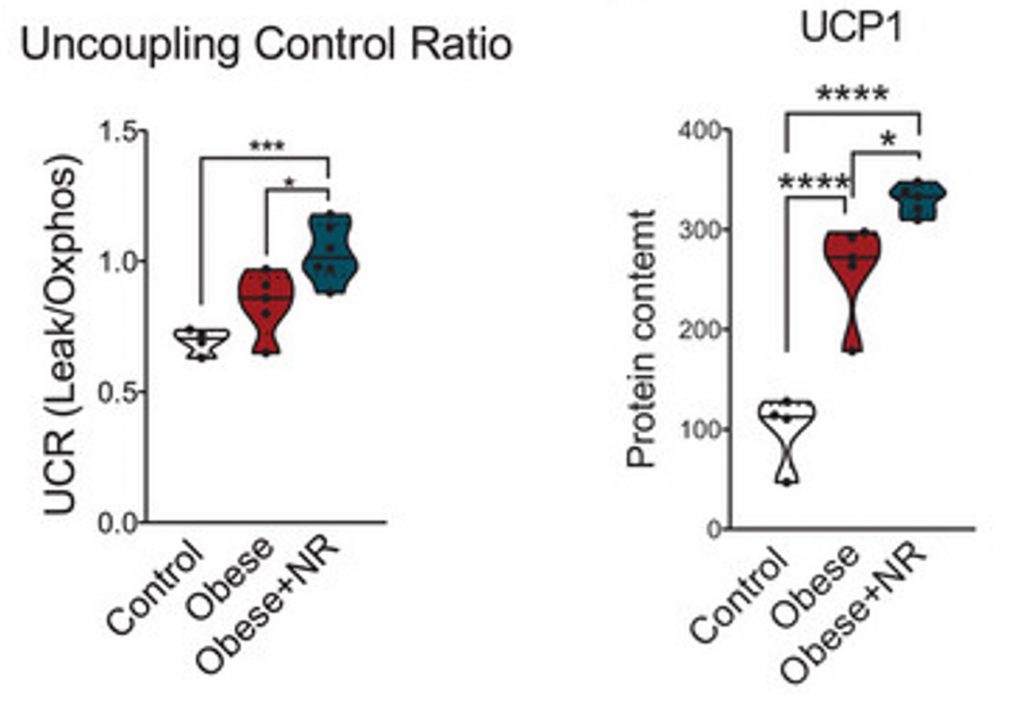
To investigate uncoupling, Ropelle and team measured the oxygen consumption rate (OCR) of brown fat tissue. Oxygen drives the movement of electrons through the ETC to generate the proton gradient needed to produce ATP or heat. They measured the OCR of brown fat tissue before and after blocking ATP-synthase. When ATP-synthase is blocked, the OCR is uncoupled from ATP production, allowing researchers to estimate uncoupling activity levels.
Based on the OCR measurements, the researchers found that treating obese mice with NR increases uncoupling. Moreover, it was shown that NR increases UCP-1 protein levels. UCP-1 protein levels were also higher than normal in obese mice, perhaps to compensate for reduced levels of brown fat cells. Still, NR increased UCP-1 beyond that of obese mice. These findings suggest that NR promotes uncoupling in brown fat tissue by upregulating UCP-1, which could potentially lead to weight loss and longevity.
Can NR Increase Uncoupling in Humans?
To investigate the effect of NR on uncoupling in humans, Dutch researchers previously examined brown fat cells biopsied from volunteers. They cultured the human brown fat cells in a dish and treated them with NR to find that NR increases uncoupling. Upon finding that NR increases brown fat tissue activity in human cells, the Dutch investigators conducted a human trial, giving overweight or obese, sedentary, middle-aged adults 1000 mg/day of NR for six weeks.
However, they found that NR did not increase the metabolic activity of brown fat, which they tested in response to cold stimulation. The researchers speculated that taking a higher dose of NR for a longer duration could lead to increased brown fat activity, or that other forms of stimulation could induce an increase in brown fat activity. Thus, more studies are needed to determine if NR can increase uncoupling in humans. In the meantime, mild cold exposure (16°C) may increase uncoupling in human muscle and brown fat tissue.