Key Points:
- NAD+ boosters are directly involved in circadian rhythms at the molecular level.
- Urolithin A may contribute to healthy circadian rhythms by improving mitochondrial health.
- Polyamines, like spermidine, may shorten circadian cycles, which become prolonged with age.
Circadian rhythm maintenance comes down to adopting a daily routine, whereby the foremost aspects of life, particularly sleeping, eating, and engaging in physical activity, are done within the same window of time. Light is the most crucial of these circadian-entraining cues, suggesting the ideal routine is aligned with the rising and falling of the sun (or with synthetic light). Following a routine and aligning life with light may improve mental and physical well-being.
The consequences of a dismantled routine can be dire, potentially leading to the development of cancer, as well as neurodegenerative, metabolic, and cardiovascular diseases. Thus, scientists now recognize the breakdown of circadian rhythms as a driving force behind aging. Yet unlike chronological time, the circadian system remains surprisingly malleable. Recent research has identified specific molecules that may restore the rhythmic precision that fades with age.
Supplements that May Counter Circadian Decline
The body runs on two types of time. One moves forward inexorably—the linear march of aging. The other circles endlessly—the 24-hour rhythm of the internal clock. For most of life, these temporal forces work in harmony. But as the years go by, rhythmicity falters, taking away the precise timing of vital functions, including hormone release, coordinated metabolism, synchronized immune function, and scheduled DNA repair.
At the molecular level, the circadian clock operates through waves of genes switching on and off, creating rhythms that cascade through every cell. Remarkably, up to 43% of genes are under circadian control. At the helm of these oscillating genes are the proteins BMAL1 and CLOCK, which orchestrate thousands of biological processes to determine the timing of life’s vital functions, such as when the liver metabolizes fats, the pancreas releases insulin, and neurons consolidate memories.
NAD+ Boosters
The temporal organization of gene expression and subsequent biological processes depends critically on NAD+ (nicotinamide adenine dinucleotide), a coenzyme involved in over 500 biochemical reactions. NAD+ serves as a molecular mediator for energy production while regulating the circadian clock itself through activation of enzymes called sirtuins, particularly SIRT1, which directly influences CLOCK and BMAL1 activity.
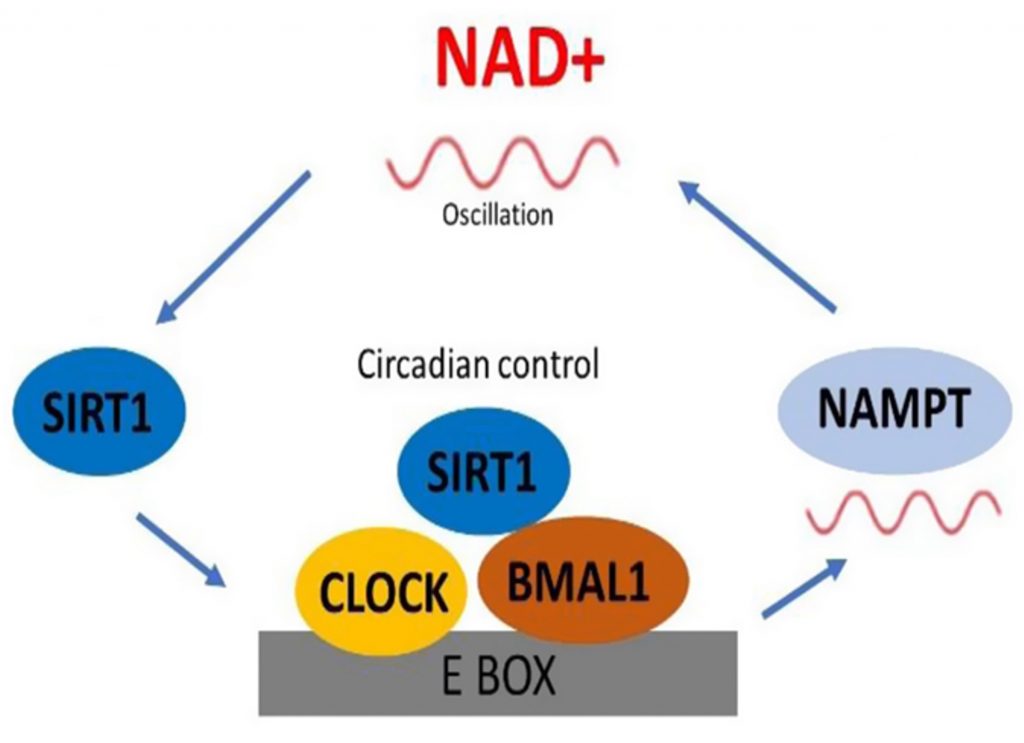
From around middle age onward, NAD+ levels steadily decline. This depletion disrupts the circadian machinery, causing peripheral clocks in organs like the liver, intestines, and nervous system to fall out of sync. Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) are examples of NAD+ precursors that cells efficiently convert to NAD+. Studies in aging mice show these compounds raise NAD+ levels to rejuvenate circadian clock gene expression.
Urolithin A
Urolithin A (UA) is a gut bacteria metabolite, produced from eating foods like pomegranates and walnuts, that declines with age. UA triggers mitophagy, the selective removal of dysfunctional mitochondria, a process that is under circadian control. Studies have shown that mitochondrial dysfunction can lead to reduced clock-dependent gene activation. Thus, improving mitochondrial health with interventions like UA may improve circadian rhythms.
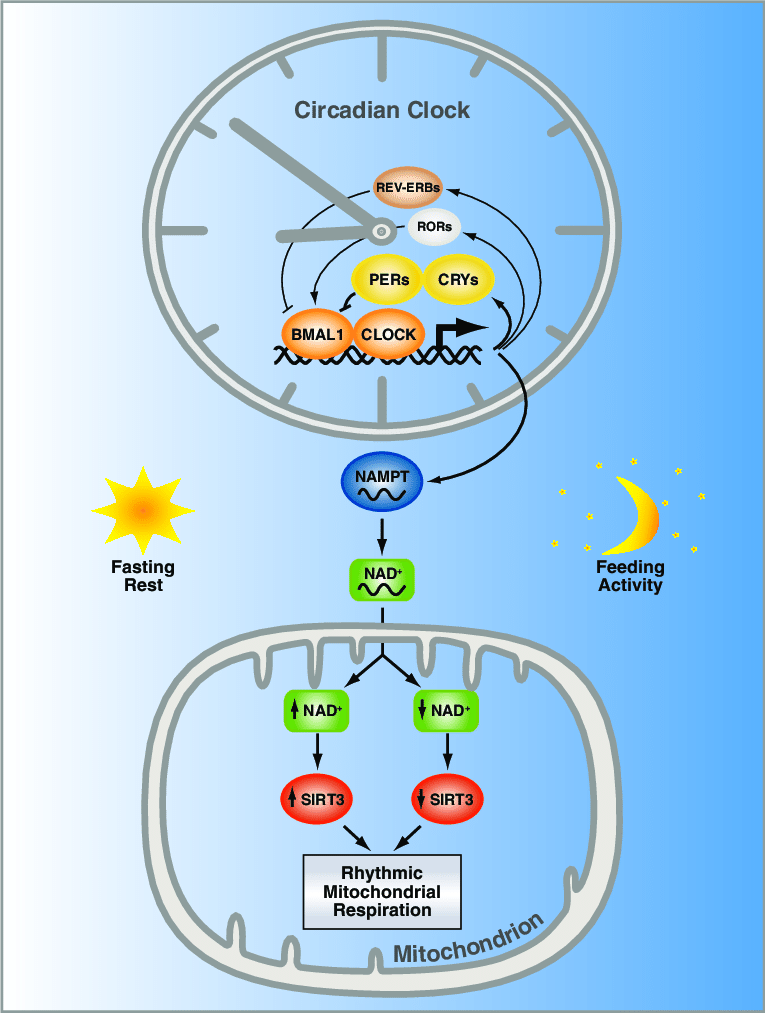
Additionally, the circadian nature of NAD+ synthesis is linked to mitochondrial function. Namely, NAD+ provides the fuel for SIRT3, a sirtuin that modulates mitochondrial respiration. Respiration is the main function of mitochondria, as it is the process leading to the production of ATP (adenosine triphosphate), the primary source of cellular energy. It follows that our circadian rhythms can control mitochondrial health, while, in turn, the health of our mitochondria can control aspects of our circadian rhythms.
Polyamines
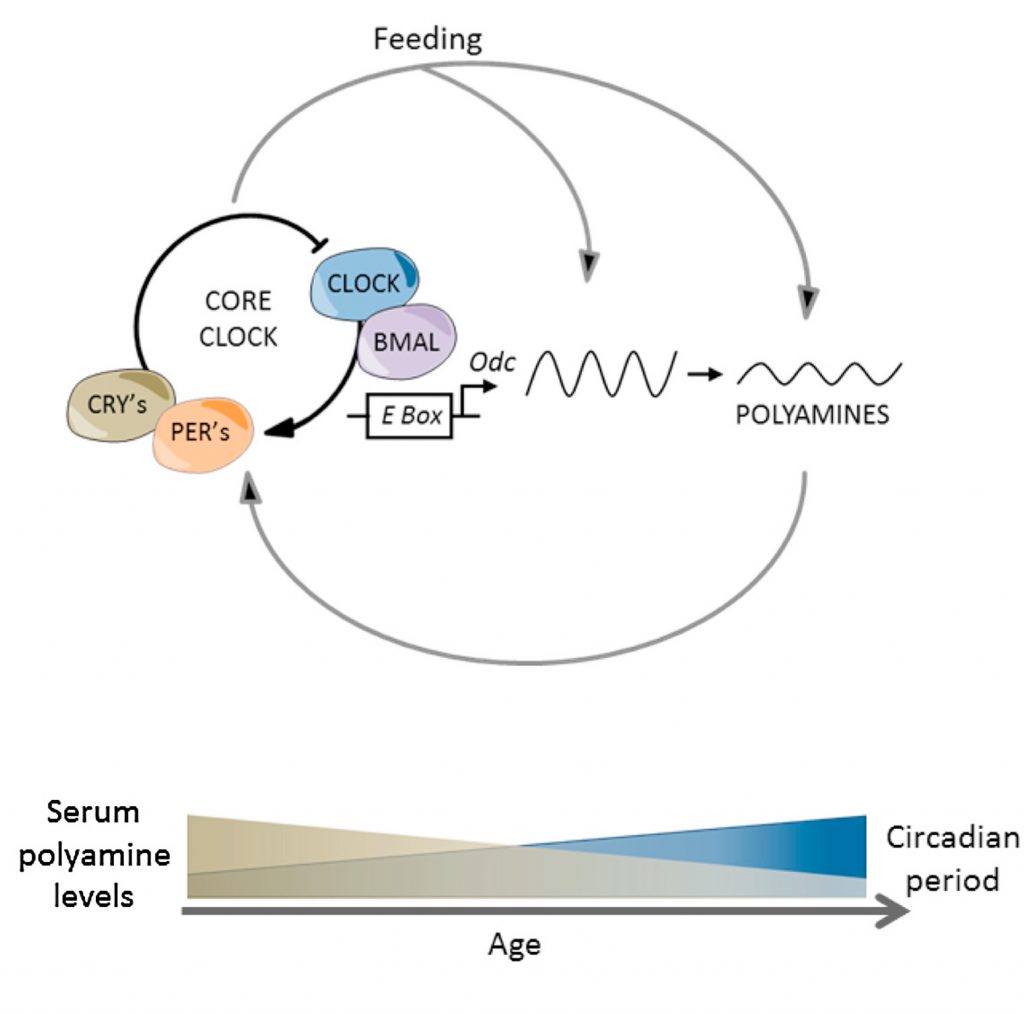
Polyamines like spermidine oscillate in concentration across the day, and, like NAD+ and UA, decline with age, correlating with various manifestations of aging. Polyamines are potent inducers of autophagy—the cellular housekeeping process that operates on a circadian schedule, ramping up during fasting periods. When polyamine levels fall, autophagy becomes sluggish and loses rhythmic precision. Damaged proteins accumulate, and the temporal separation between building and breaking down blurs.
Restoring polyamine levels reactivates autophagy and improves healthspan in animal models. Animal studies have also shown that polyamines modulate the circadian period, the duration of time it takes for each circadian cycle to complete, which in both humans and mice is around 24 hours. With age, the circadian period increases, leading to circadian rhythm disruptions. However, supplementing with polyamines, like spermidine, shortens the age-related lengthening of the circadian period by replenishing polyamine levels.
Together, NAD+, UA, and polyamines work through distinct mechanisms yet converge on shared pathways undergirding circadian function. NAD+ directly powers clock machinery. UA may help ensure that mitochondria remain synchronized. Polyamines may contribute to maintaining circadian schedules. Together, they may address “circadian aging,” when the circadian system weakens, temporal coordination is lost, and thousands of processes occur at the wrong time.
Supplements are Supplements
It’s important to remember that supplements are meant to supplement lifestyle factors and, under optimal circumstances, circadian health can be achieved without supplements. Consistent sleep-wake schedules, strategic light exposure (bright mornings, dim evenings), time-restricted eating (10-12 hour feeding windows during the day), regular activity, and social engagement remain the most powerful interventions.
However, contemporary life relentlessly attacks circadian health through artificial light at night, irregular eating, social jetlag, shift work, screens, sedentary lifestyles, and social isolation. These sustained assaults weaken the internal clock’s amplitude and increase vulnerability to age-related disease. Under these suboptimal conditions, it is possible that supplements may provide some leeway.