Key Points:
- The mTOR pathway is involved in modulating growth, sometimes at the expense of longevity.
- Long-lived individuals have genetic mutations in the mTOR pathway that may optimize for growth and longevity.
In a new study published in GeroScience, German researchers have uncovered compelling evidence that a cellular pathway called mTOR, often described as the body’s “longevity switch,” may hold the key to understanding how we age and how to extend healthy lifespan.
What Is the mTOR Pathway?
The mechanistic target of rapamycin (mTOR) signaling pathway serves as a master regulator of cellular metabolism, growth, and survival. Its regulatory roles include:
- Protein synthesis: mTOR initiates the translation of mRNA into proteins.
- Autophagy: mTOR reduces autophagy, the process cells use to degrade and recycle cellular components, such as proteins.
mTOR is activated by various molecules, including insulin and amino acids, especially leucine. In other words, mTOR is activated when we eat foods containing carbohydrates (triggering insulin release) and proteins, which contain amino acids like leucine. In this context, mTOR activity decreases during periods of fasting.
Rare mTOR Pathway Genes in Long-Lived People
The German researchers sought to elucidate why people live well into their 90s or even past 100, and others do not. While lifestyle factors like diet and exercise certainly play important roles, research on long-lived families has revealed that genetics contributes significantly to longevity, accounting for up to 50% of the variation in lifespan.
Taking an innovative approach, the researchers focused on rare genetic variants, present in less than 1% of the population, rather than common variants, which were examined previously. They analyzed the protein-coding regions of DNA (exons) from 1,245 long-lived Germans (average age 98.9 years, range 94–110 years) and 4,105 younger controls (average age 50.5 years, range 18–83 years).
The results were striking: three rare gene variants involved in the mTOR pathway — RPS6, FLCN, and SIK3 — were more common in the long-lived individuals. These findings may lead to studies examining the role of these genes in promoting longevity.
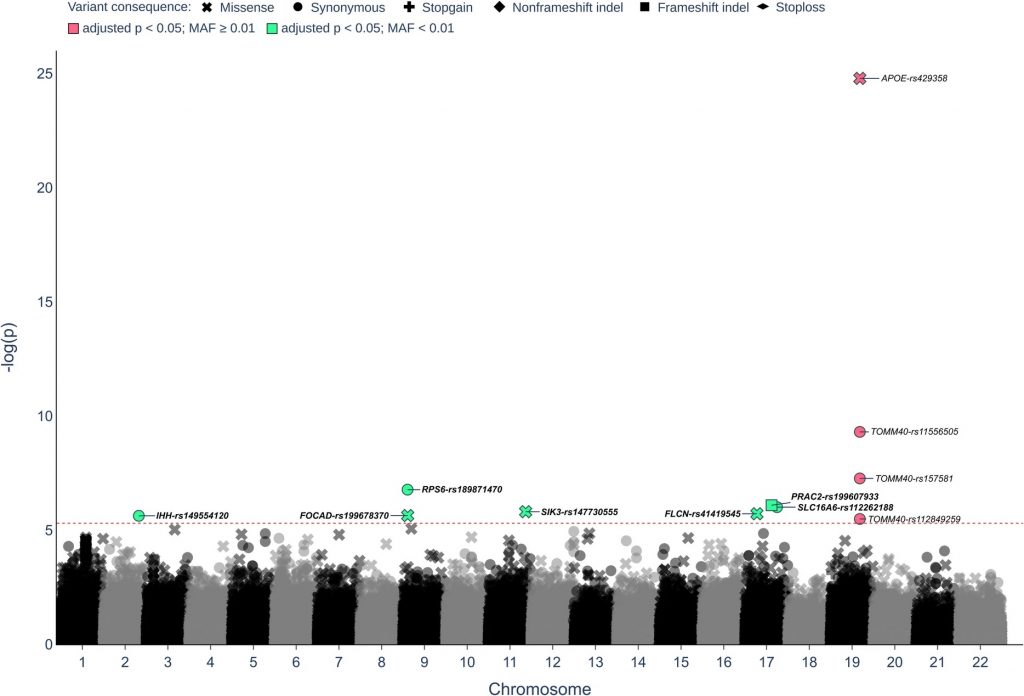
mTOR Pathway Complexity
The mTOR pathway is complex and still under intense investigation. At the heart of the pathway is mTOR, which is part of mTOR complex 1 (mTORC1). Many studies support the idea that inhibiting mTORC1 promotes a longer life. Since the normal variants of FLCN and SIK3 are known to be involved in activating mTORC1, the researchers speculate that the long-lived variants do the opposite by reducing mTORC1 signaling. Supporting this prediction, the loss of FLCN has been shown to induce longevity in worms, alluding to the long-lived variant of FLCN exhibiting lost functionality.
Compared to FLCN and SIK3, more is known of the RPS6 gene, which produces a protein called S6. S6 is involved in protein synthesis and cell proliferation. Thus, the long-lived variant of RPS6 may alter S6 in a way that promotes longevity. Genetically deleting S6 kinase, which activates S6, was shown to extend the lifespan of the mice, suggesting that the long-lived version of S6 could be less active. Indeed, numerous studies have shown that reducing protein synthesis, namely protein translation, prolongs lifespan. Overall, it would seem that the long-lived variants of FLCN, SIK3, and RPS6 suppress different parts of the mTOR signaling pathway.
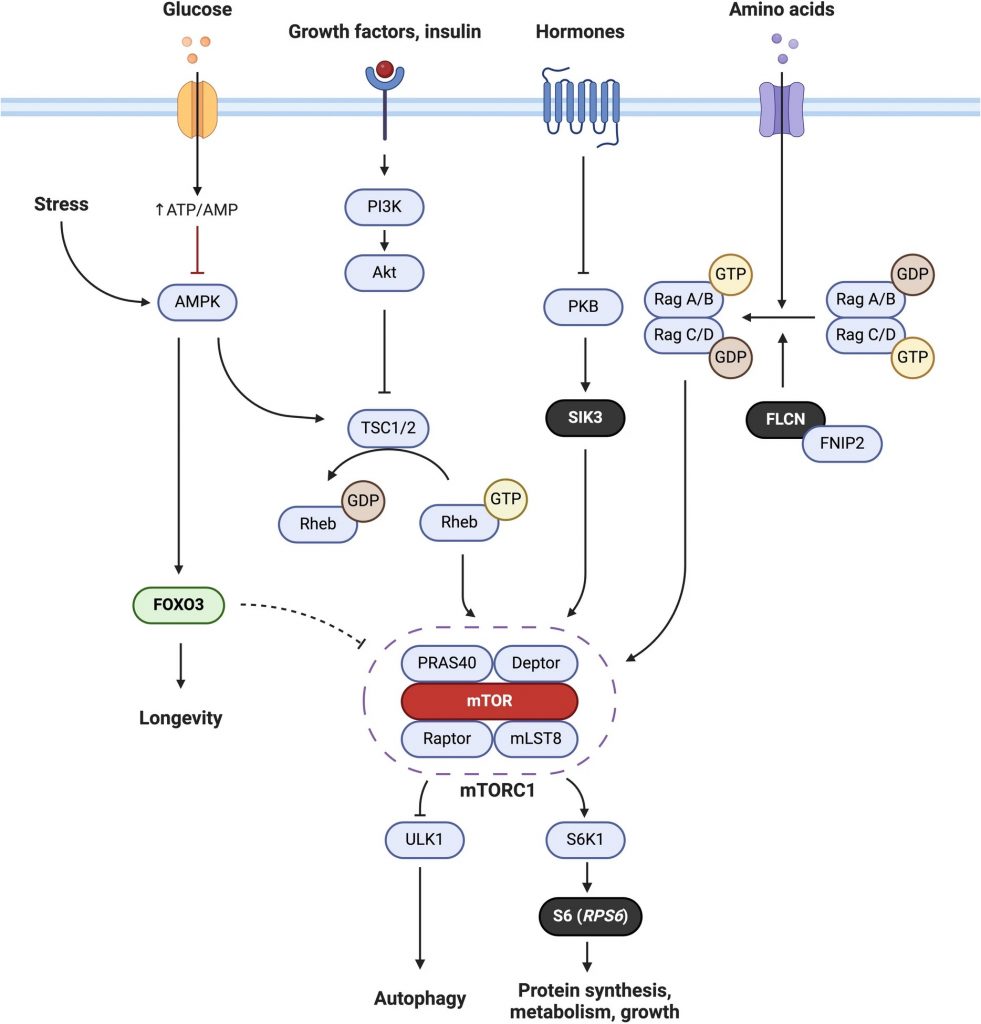
Inhibiting mTOR to Slow Aging
As stated above, reducing the efficacy of certain components of the mTOR signaling pathway, such as FLCN and S6, leads to a longer life in animal models. Additionally, inhibiting mTOR with the prescription drug rapamycin has proved to be one of the most potent single-compound interventions for extending animal lifespan. Findings like these have led scientists to proclaim that mTOR is a central regulator of lifespan of aging.
However, complete inhibition of mTOR is incompatible with life. What we may be seeing in long-lived individuals is a recalibration of the mTOR pathway that may optimize the balance between growth and maintenance. The long-lived individuals in the study appear to have genetic variants that naturally achieve this optimal balance.
This suggests that future interventions targeting mTOR for healthy aging would need to be precisely calibrated, not shutting down the pathway entirely, but adjusting its activity in specific ways. The rare genetic variants identified in the German study provide valuable clues about how such fine-tuning might be achieved.
Future Research
The discovery that rare genetic variants in the mTOR pathway are associated with exceptional longevity opens several promising avenues for future research and potential interventions:
- Developing more targeted mTOR modulators: Rather than broadly inhibiting mTOR like rapamycin does, researchers might develop compounds that affect specific components of the pathway, mimicking the effects of the beneficial genetic variants.
- Personalized approaches based on genetic profiles: Understanding how individual genetic variations affect mTOR signaling could lead to personalized interventions tailored to each person’s unique genetic makeup. Someone with naturally lower mTOR activity might need different interventions than someone with higher baseline activity.
- Lifestyle interventions that optimize mTOR: Certain dietary patterns — particularly intermittent fasting and caloric restriction — are known to modulate mTOR activity. The genetic findings could help researchers develop more targeted lifestyle recommendations that optimize mTOR signaling.
- Combining mTOR modulation with other approaches: The most effective longevity interventions might involve targeting mTOR alongside other aging-related pathways, creating synergistic effects that address multiple aspects of the aging process simultaneously.