Key Points:
- Mice introduced to a simulated natural environment age faster than their genetically identical counterparts in the lab.
- For humans, there are ways of mitigating the age-accelerating effects of living like a mouse.
With the comforts and conveniences of modern society, are we more like wild mice or lab mice? By comparing lab mice with their “rewilded” counterparts living in semi-natural outdoor environments, researchers have uncovered striking differences in how quickly their biological clocks tick. The findings give us clues on when to be comfortable and when to enter survival mode in the context of living a longer and healthier life.
Simulating Natural Life
Laboratory conditions are almost nothing like the environments in which mice evolved to inhabit. In nature, mice face fluctuating temperatures, diverse social interactions, competition for resources, and the constant vigilance required to avoid predators. In a collaboration between Cornell University and the University of Pennsylvania (UPenn), researchers sought to understand how these ecological realities affect aging at the molecular level.
To investigate this question, the research team took genetically identical mice (the common C57BL/6J laboratory strain used in countless studies worldwide), injected them with radio-frequency identification (RFID) tags, and released some as infants into specially designed outdoor enclosures at Cornell University. These “rewilded” mice experienced natural weather patterns, complex social interactions, competed for resources, and navigated a physical environment 9,000 times larger than a standard laboratory cage.
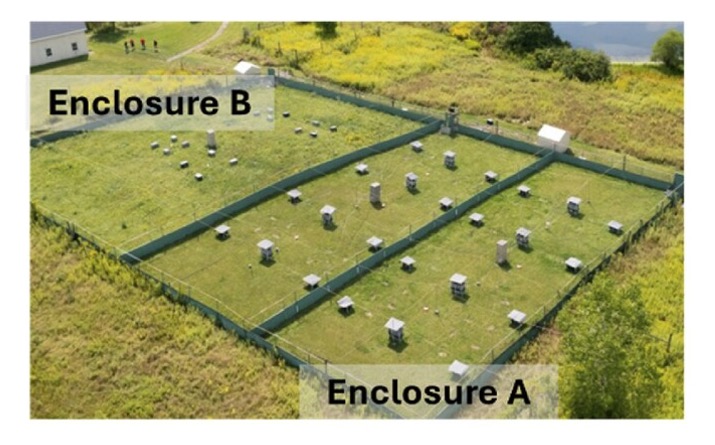
Meanwhile, genetically identical mice remained in standard laboratory housing, with consistent temperature, regular feeding schedules, and limited social complexity. This experimental design created a powerful natural experiment: genetically identical animals experiencing radically different environments.
Measuring Biological Time
To measure aging, the researchers examined what scientists call epigenetics—chemical modifications to DNA that don’t change the genetic code itself but control how genes are expressed. These modifications, particularly a process called DNA methylation, change with age and can serve as a kind of molecular timekeeper. For this reason, age-related epigenetic changes are increasingly employed to measure biological aging, as they can predict age-related disease and mortality risks.
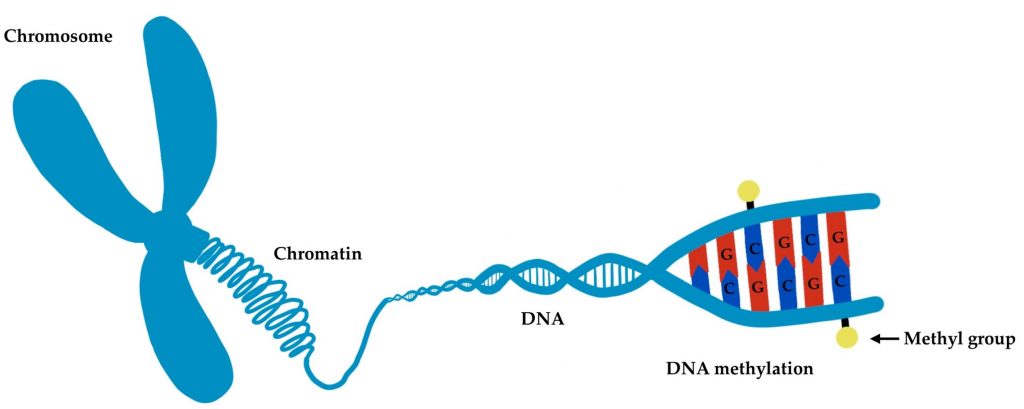
DNA methylation can either increase or decrease. Hypo-methylation describes DNA sites with reduced DNA methylation. Overall, aging is associated with increased hypomethylation. In contrast, hyper-methylation describes DNA sites with increased DNA methylation.
Rewilded Mice Age Faster than Lab Mice
After collecting liver tissue samples from both lab and rewilded mice, the researchers analyzed approximately 275,000 sites in the genome where methylation occurs. The epigenetic aging rate of the mice was determined by the change in DNA methylation per year. The results showed that both lab and rewilded mice exhibit many similar epigenetic changes with age. These initial observations suggest that intrinsic factors lead to epigenetic changes regardless of the environment. However, there were still important environment-dependent differences.
As expected, hypomethylation increased in all mice due to aging. This is consistent with the overall decline in DNA methylation that occurs with aging. Still, hypermethylation was elevated in rewilded mice compared to lab mice.
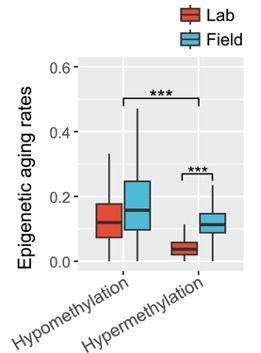
Moreover, of the hypermethylation sites known to be sensitive to aging, where DNA typically gains methylation with age, epigenetic aging was approximately 94% faster in rewilded mice.
Additionally, of the age-associated hypomethylation sites, where DNA typically loses methyl groups with age, the epigenetic aging rate of the rewilded mice was approximately 28% faster. These findings reveal that mice living in a more natural outdoor environment exhibit significantly faster epigenetic aging than their laboratory counterparts.
Notably, the researchers also applied “epigenetic clocks”—statistical models that predict biological age based on methylation patterns—to their samples. These clocks consistently overestimated the chronological age of field mice compared to lab mice, further confirming the accelerated aging phenomenon.
Consequences of Epigenetic Aging
Epigenetic changes trigger aging by turning genes on and off, so the consequences of epigenetic aging depend on which genes are affected. The researchers found that genes involved in metabolism and DNA repair were particularly pronounced in rewilded mice compared to lab mice. For instance, sites associated with a transcription factor called ISL1, which regulates insulin and fat metabolism, showed accelerated methylation changes in field mice.
The researchers also found that mice introduced to the outdoor environment as adults showed different patterns of accelerated aging compared to those “rewilded” as infants. For example, the adult mice showed accelerated methylation at sites containing DNA damage repair genes. This suggests that early-life exposure to environmental complexity may program aging trajectories differently than exposure later in life, a finding with potential implications for understanding human development as well.
What It Means for Humans
Do the comforts and conveniences of post-industrial living make us age faster or slower than our pre-civilization “wild” ancestors? The answer may lie in how often we encounter and deal with stress. We were born into a society with increasingly controlled, comfortable environments that greatly reduce our chances of encountering stress. Examples include:
- Temperature-controlled enclosures (e.g., homes, buildings, vehicles) protect us from weather changes.
- The mass production and distribution of food relieves us from the stress of hunting, gathering, or farming.
- The reduced need for physical exercise due to the luxuries of modern society mentioned above.
In this respect, we are more like lab mice that are sedentary and live in a temperature-controlled cage with unlimited access to food.
However, we now know that sedentary people who freely consume food to the point of becoming obese are at higher risk for age-related diseases (e.g., cardiovascular disease, neurodegenerative diseases) and early mortality. Therefore, the comforts of modern society do not seem to make us age more slowly, but appear to make us age faster.
It is well established that consuming fewer calories and regularly exercising lowers the risk of age-related diseases and potentially increases the chances of living a longer life. In this respect, we can simulate the lifestyles of our wild ancestors by engaging in regimented physical activity and limiting our access to food to reduce our rate of aging.
Still, when it comes to social complexity, we are more like wild mice that have to compete for resources and mates. This social complexity may influence our survival, as access to resources can determine whether we have access to food. Furthermore, the psychological stress induced by social complexity may be exacerbated by the physical stress of a sedentary lifestyle combined with consuming excess calories.
However, research also shows that maintaining healthy social connections is conducive to living a longer and healthier life. It could be that maintaining healthy social relationships mitigates the stress of social complexity.
Overall, it would seem that we have similarities with both lab mice and wild mice. Nevertheless, since we are not mice, we have the opportunity to control our environment to a certain extent. We can partake in science-backed interventions that increase our chances of living longer without disease. These interventions include limiting the consumption of excess calories, exercising regularly, and maintaining fulfilling social connections.