Key Points:
- Rapamycin prevents cell death from DNA damage in human immune cells.
- In older adults, higher mTOR activity is linked to higher levels of DNA damage.
- Rapamycin supplementation reduces a marker for senescence in the immune cells of older adults.
Scientists are continually uncovering fascinating molecular pathways that govern the aging process. Among the most promising discoveries is rapamycin, a compound that has garnered significant attention for its remarkable ability to extend lifespan in various organisms. A new study (not yet peer-reviewed) from Oxford University sheds light on a critical aspect of its lifespan-extending power: healing the human immune system.
Rapamycin: An mTOR Inhibitor
Rapamycin was discovered in a volcano crater on Easter Island, also known as Rapa Nui, in 1964. It is now known that rapamycin inhibits mTOR (mammalian target of rapamycin), a central regulator of cell growth that acts as a nutrient sensor, dictating whether cells should divide or enter a state of repair known as autophagy. Today, mTOR inhibition is considered rapamycin’s primary mechanism of action.

The FDA approved Rapamycin as an immunosuppressant drug in 1999. Still, it wasn’t until 2009 that it entered the realm of aging biology research, when it was shown to extend the lifespan of both male and female mice. This was a keystone discovery, as it was the first time a pharmacological agent was shown to extend the lifespan of mammals.
By dampening mTOR activity, rapamycin shifts cellular processes towards maintenance and repair, mimicking the effects of caloric restriction, a well-established longevity intervention.
Studies in diverse organisms have consistently shown that rapamycin not only extends lifespan but also delays the onset of age-related diseases. These effects are attributed to its influence on various cellular hallmarks of aging, including DNA damage and cellular senescence.
Cellular Senescence: A Driver of Aging
To appreciate rapamycin’s impact, it is worth delving deeper into the concept of cellular senescence. These are cells that, instead of dying upon being damaged, stop dividing. Moreover, they secrete a cocktail of molecules that contribute to chronic low-grade inflammation, disrupt tissue function, and promote biological aging. Senescent cells accumulate with age in nearly all tissues, including the immune system.
Rapamycin Prevents Cell Death from DNA Damage
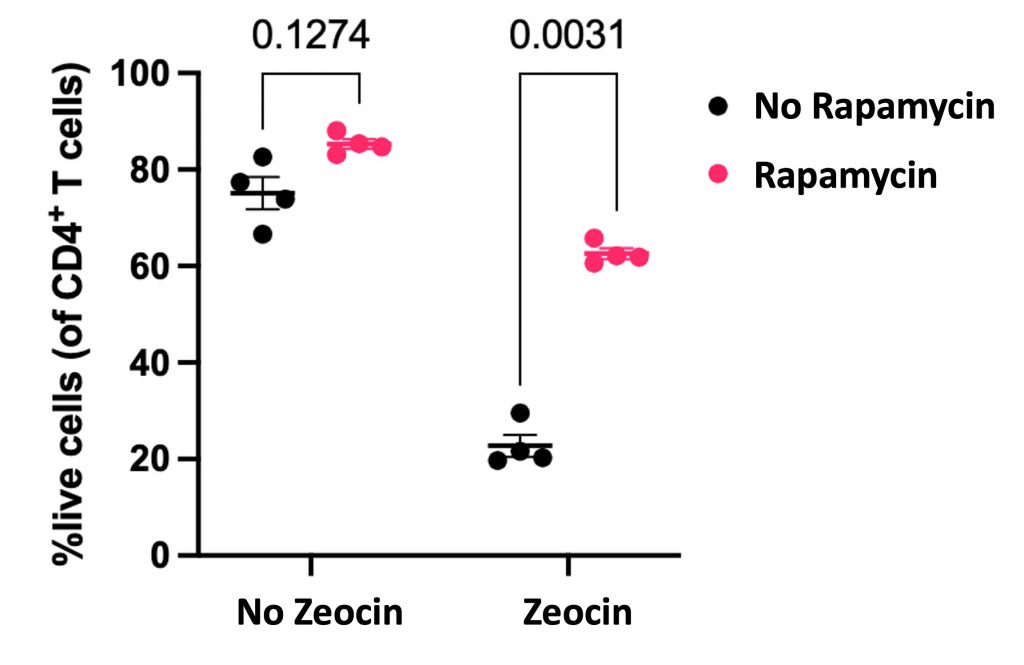
To determine how inhibiting mTOR affects the immune system, the Oxford researchers examined human T cells. T cells are white blood cells that protect the body from infections and cancer. By treating T cells with rapamycin, the researchers found that this mTOR inhibitor prevented DNA damage induced by an antibiotic known as zeocin. Moreover, rapamycin was shown to increase the survival rate of T cells exposed to zeocin by 3-fold, suggesting it can protect the immune system from DNA damage and deterioration.
Rapamycin Reduces Senescent Immune Cells in Humans
The Oxford researchers next conducted a pilot clinical study consisting of older male volunteers. The volunteers received either 1 mg/day of rapamycin or a placebo for 8 weeks. The 1 mg/day rapamycin dose is considered a low dose, which was shown to enhance immune function and reduce infections in the elderly. In other words, low-dose rapamycin appears to have the opposite effect of high-dose rapamycin, which is an immunosuppressant. Indeed, the rapamycin groups showed no reduction in white blood cells, suggesting no immunosuppression.
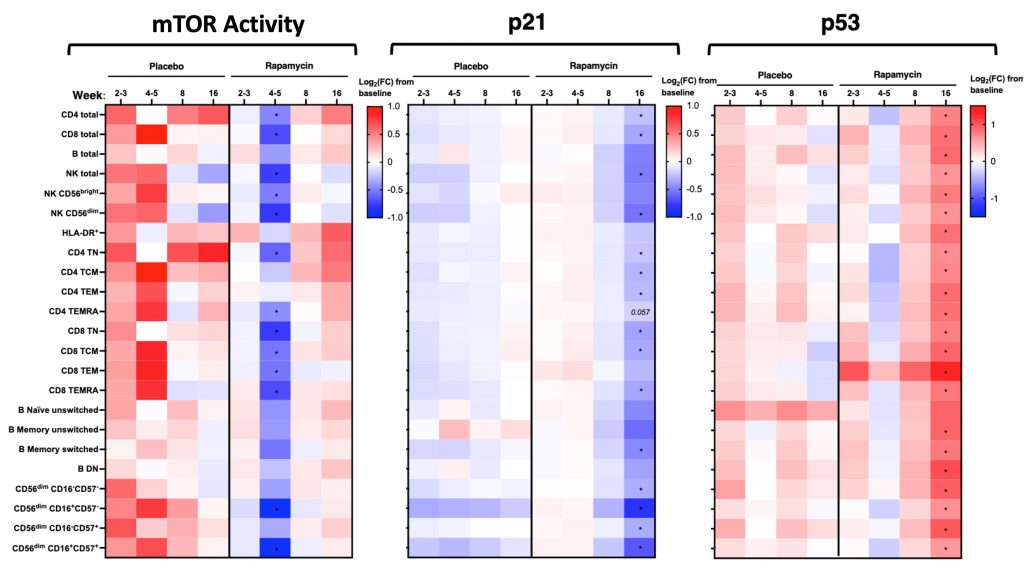
Curiously, the researchers found that their marker for mTOR activation (p-S6) was reduced only during weeks four and five of supplementation. Still, in examining immune cell DNA damage, the researchers found a strong correlation between mTOR activation and DNA damage in both treatment groups, suggesting mTOR activity and DNA damage are linked. Moreover, the rapamycin group had lower levels of a senescence marker called p21 in most of their immune cells. However, the p53 senescence marker was higher in the rapamycin group. To this, the authors said,
“Though at this stage highly speculative, it is possible that elevated p53 in immune cells from rapamycin-treated participants may indicate better overall metabolic health.”
Human Evidence for mTOR Inhibition’s Longevity Effects
The Oxford study adds to a growing body of studies testing the effects of rapamycin on humans. In a recent publication, physicians reviewed 19 studies testing the effects of mTOR inhibitors on age-related physiological changes and disease. Some of the benefits of mTOR inhibition include:
- Reduced senescent markers in the skin
- Reduced cortisol levels
- Increased vaccine effectiveness against the flu and respiratory tract infections
- Mitigation of arthritis when combined with conventional treatment
- Improved cardiovascular function in people with pulmonary hypertension
The adverse effects of mTOR inhibition found in these studies include an increased number of infections, increased LDL cholesterol levels, and increased triglyceride levels. Other complications in both humans and mice include testicular atrophy and glucose intolerance. Corroborating these effects, the biohacker Bryan Johnson recently stopped taking rapamycin due to elevated blood glucose, susceptibility to infection, and impaired healing.
Based on the human studies, rapamycin may be most beneficial for older adults with conditions such as arthritis or pulmonary hypertension. As far as using mTOR inhibition to prevent age-related disease and live longer, the complexities of biological aging make it difficult to determine whether mTOR inhibition alone is effective.