Key Points:
- In genetically diverse male mice, researchers found that canagliflozin treatment enhances learning and memory.
- Canagliflozin enhances the function of the cell’s powerhouse (mitochondria) and suppresses neuroinflammation in male but not female mice.
- Further experimentation using an Alzheimer’s disease mouse model revealed that canagliflozin reduces the burden of Alzheimer’s-associated plaques.
As published in a non-peer-reviewed preprint, Sadagurski and colleagues from Wayne State University in Detroit show that the diabetes drug canagliflozin enhances learning and memory in middle-aged male mice. Further analyses of canagliflozin’s effects on gene expression, protein levels, and metabolic markers revealed that canagliflozin enhances mitochondrial function and suppresses neuroinflammation in middle-aged males but not females. Moreover, additional experimentation using an Alzheimer’s mouse model showed that canagliflozin reduced the burden of Alzheimer’s-associated plaques in the brains of males. These findings suggest that canagliflozin confers sex-specific effects against cognitive decline with age and may counteract molecular features associated with Alzheimer’s disease progression.
Researchers have previously shown that canagliflozin extends the average lifespan of mice, in males but not females, by 14%. The same study showing that canagliflozin extends male mouse lifespan also suggested that the lifespan extension came from lowered blood sugar levels. In a similar vein, elevated blood sugar has been linked to dementia. As such, Sadagurski and colleagues sought to examine whether canagliflozin can, in addition to extending male mouse lifespan, counteract cognitive decline through its blood sugar-lowering effects.
Canagliflozin Improves Learning and Memory
To assess how canagliflozin affects cognition, Sadagurski and colleagues treated middle-aged mice with the drug, starting at seven months of age (roughly equivalent to 33-year-old humans). Then, at ages 12 to 14 months (roughly between the ages of 44 and 48 for humans), the researchers tested their cognitive abilities. To test cognition, Sadagurski and colleagues evaluated spatial learning and memory with a test called the Barnes maze.
The Barnes maze consists of a circular platform with holes around its perimeter, one of which allows a mouse access to an escape box. After four days of training, the researchers tested the mice’s abilities to remember the target hole that allows access to the escape box. On days following the four-day training period, canagliflozin-treated males located the target hole significantly faster than non-treated mice. These results suggest that canagliflozin enhances learning and memory abilities for males.
In an effort to get a better idea of how canagliflozin improves cognition at the molecular level, Sadagurski and colleagues analyzed gene expression, protein levels, and metabolic features of the genetically diverse mice treated with canagliflozin. The analyses revealed that, compared to non-treated mice, canagliflozin-treated males exhibited markers of enhanced mitochondrial function as well as suppressed neuroinflammation. These data suggest that canagliflozin improves mitochondrial function in neurons and lowers neuroinflammation to enhance brain function and subsequently, learning and memory.
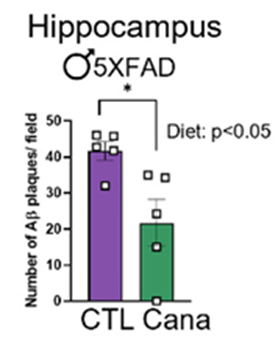
To find whether canagliflozin may work against Alzheimer’s dementia, Sadagurski and colleagues utilized an Alzheimer’s disease mouse model. In this mouse model, they found that canagliflozin reduced the quantity of beta-amyloid plaques associated with Alzheimer’s disease in a brain region with crucial roles in learning and memory, the hippocampus, in males only. This finding supports that canagliflozin counteracts the buildup of plaque in the brain associated with Alzheimer’s dementia in males.
Sadagurski and colleagues then sought to find whether canagliflozin improves cognition in the Alzheimer’s mouse model. To assess spatial memory, Sadagurski and colleagues utilized the Y-maze—a maze shaped like the letter Y. Generally, mice with normal short-term memory capabilities will preferentially examine unexplored arms of the Y-maze, so they will alternate between different arms more frequently. Accordingly, alternating between arms indicates that mice remember they have explored certain arms and moved on to other arms of the maze.
Canagliflozin-treated male Alzheimer’s model mice performed significantly better than non-treated Alzheimer’s model mice at alternating between arms of the Y-maze. Moreover, the canagliflozin-treated males performed similarly to typical, non-Alzheimer’s model mice at the Y-maze task. This finding suggests that canagliflozin rescues memory in male Alzheimer’s model mice.
“Together, our findings establish Canagliflozin as a promising gerotherapeutic candidate for [Alzheimer’s disease] prevention…” said Sadagurski and colleagues in their publication.
Figuring Out Whether Canagliflozin Counteracts Cognitive Decline in Humans
This study from Sadagurski and colleagues suggests that the FDA-approved diabetes medication canagliflozin protects against brain aging, possibly by reducing blood sugar levels. In that sense, canagliflozin enhanced learning and memory in genetically diverse, middle-aged male mice and even prevented cognitive deterioration in male Alzheimer’s model mice.
Furthermore, there are already FDA-approved drugs that work similarly to canagliflozin, which, along with canagliflozin, are classified as SGLT2 inhibitors and may confer similar effects. As such, further research to gain more detailed mechanistic insights into canagliflozin’s course of action may provide clues as to which SGLT2-inhibiting drugs could counteract Alzheimer’s dementia in males.
Sadagurski and colleagues’ experiments were done in mice, so uncovering whether canagliflozin’s effects against Alzheimer’s apply to humans will need human trial testing. Currently, there is no data definitively linking the use of SGLT2 inhibitors like canagliflozin to a reduced risk of Alzheimer’s dementia in healthy aging adults. All the same, there was a study suggesting SGLT2 inhibitors reduce the risk of dementia in diabetes patients. If further data comes to light showing an association between taking canagliflozin and a reduced risk of Alzheimer’s dementia, figuring out optimal dosing and duration for taking canagliflozin will be necessary.
Finally, a downside to Sadagurski and colleagues’ study was that canagliflozin’s cognition-preserving effects only applied to males. Accordingly, it still remains crucial to find ways to counteract age-related cognitive decline in females. However, since canagliflozin’s sex-specific cognition-preserving effects were shown in mice, there is still a possibility that the drug could help preserve cognition in both aging human males and females. Altogether, quite a bit of research needs to be done to demonstrate that canagliflozin counteracts age-related cognitive decline in people, yet the fact that the drug is already FDA-approved could make studying canagliflozin easier.

