Key Points:
- “Super stem cells” improve the memory of monkeys while protecting against neurodegeneration.
- The super stem cells prevent age-related bone loss while rejuvenating over 50% of the 61 tissues analyzed.
- Treatment with stem cells reduces inflammation and senescent cells (cells that accumulate to promote aging).
While small in number, our adult stem cells play a crucial role in regenerating our lost or damaged tissues, rebuilding our body cell by cell. However, with age, our bodies become riddled with inflammation, hardly providing an environment capable of keeping our stem cells healthy. Eventually, our stem cells lose their regenerative capacity, contributing to degenerative aging.
Fox, O, Three
Hydra are a genus of immortal beings that live forever in freshwater environments like lakes and ponds. What scientists have called “nothing but an active stem cell community,” Hydra can escape death by infinitely regenerating. Their stem cells can continuously proliferate and renew by producing FoxO, a protein they share with humans.
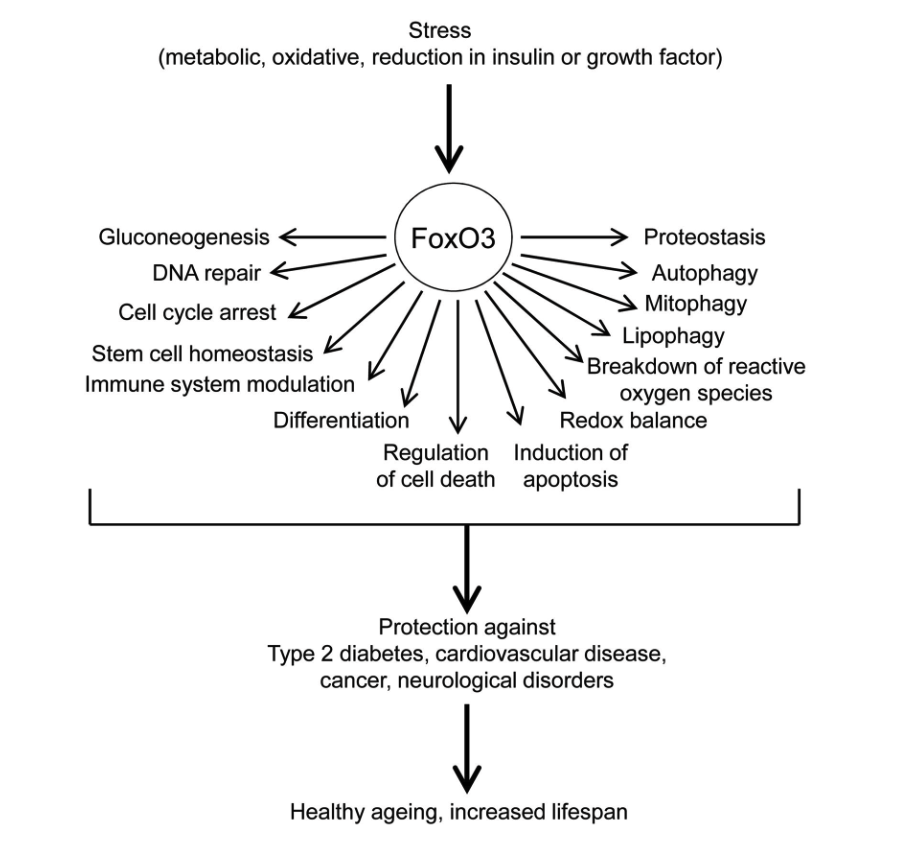
In humans, the FoxO protein, specifically the FoxO3 isoform, responds to cellular stress by binding to DNA and turning genes on and off. These genes are involved in numerous cellular processes that promote healthy aging and extended lifespan. This is why the FoxO3 protein’s corresponding gene, FOXO3, is considered a longevity gene.
Experimenting with SRCs
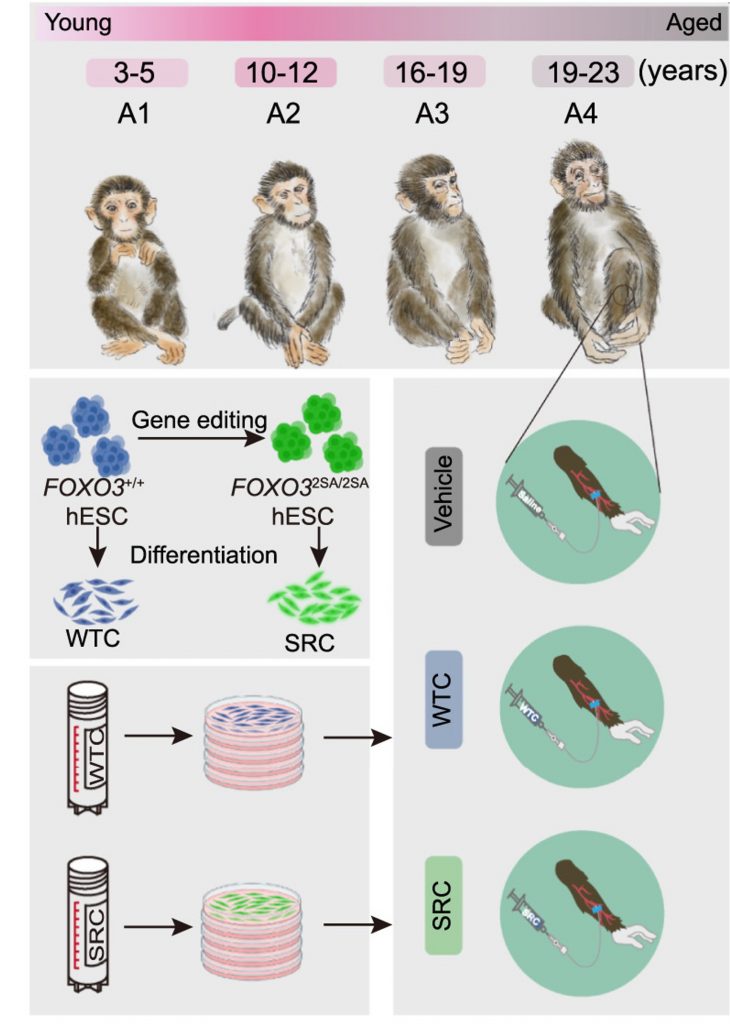
As FoxO3 assists cells in resisting stressful environments, such as inflamed tissue, Chinese Academy of Sciences researchers engineered human stem cells to have enhanced FoxO3 activity. As published in Cell, these senescence-resistant stem cells (SRCs) were designed to exhibit greater resistance to age-related stress. To test this, cynomolgus monkeys, also known as crab-eating macaques, were first stratified into four groups based on age:
- A1: 3-5 years (approximately equivalent to 9-15 human years)
- A2: 10-12 years (approximately equivalent to 30-36 human years)
- A3: 16-19 years (approximately equivalent to 48-57 human years)
- A4: 19-23 years (approximately equivalent to 57-69 human years)
The oldest of the monkeys, the A4 group, were the focus of the study and were subdivided into three groups. One group was injected with saline (salt and water), another group with normal stem cells, and the third with SRCs. The aged monkeys were injected every two weeks for 44 weeks, approximately equivalent to the duration of three human years. On safety, there were no serious adverse events, such as immune system rejection or tumor growth.
SRCs Improve Cognition
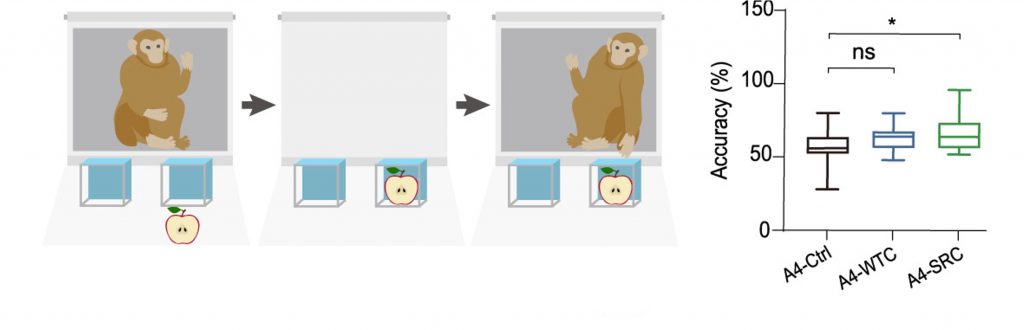
After 44 weeks of biweekly injections, a suite of biological indices was measured from the aged monkeys to assess whether SRCs slow down biological aging. One of these indices was memory retention. To assess memory, the researchers conducted a common experiment called the Wisconsin General Test Apparatus (WGTA).
For this experiment, each monkey was trained to retrieve food located outside of one of two identical boxes. During the test session, after each monkey was trained, food was placed next to one of the boxes to keep it hidden. Subsequently, a flap was placed in front of the monkey to block the boxes from view. Three seconds later, when the flap was reopened, each monkey had to remember which of the two boxes contained the food.
Remarkably, the monkeys injected with SRCs remembered the location of the food with higher accuracy than the monkeys injected with saline. Moreover, the monkeys injected with normal stem cells exhibited the same level of accuracy as the monkeys injected with saline. These findings suggest that SRCs, and not normal stem cells, improve the memory of aged monkeys.
Furthermore, MRI-based structural analysis showed that treatment with SRCs mitigated age-related brain shrinkage. MRI-based experiments also revealed that brain connectivity was restored to that of young (A1 group) monkeys. Namely, the structural connectivity between seven brain regions, including those important for working memory (prefrontal cortex), was rejuvenated with SRC treatment. Overall, these findings suggest that SRC injections improve memory by protecting against neurodegeneration.
SRCs Rejuvenate Many Organs and Tissues
In addition to the brain, the Academy researchers found that SRC treatment rejuvenated multiple organs and tissues. This is important because the rejuvenation of a given organ or tissue could lead to the reduced risk of its corresponding age-related chronic diseases. For example, the rejuvenating effects of SRCs on the brain could reduce the risk of neurodegenerative disorders like Alzheimer’s and Parkinson’s diseases.
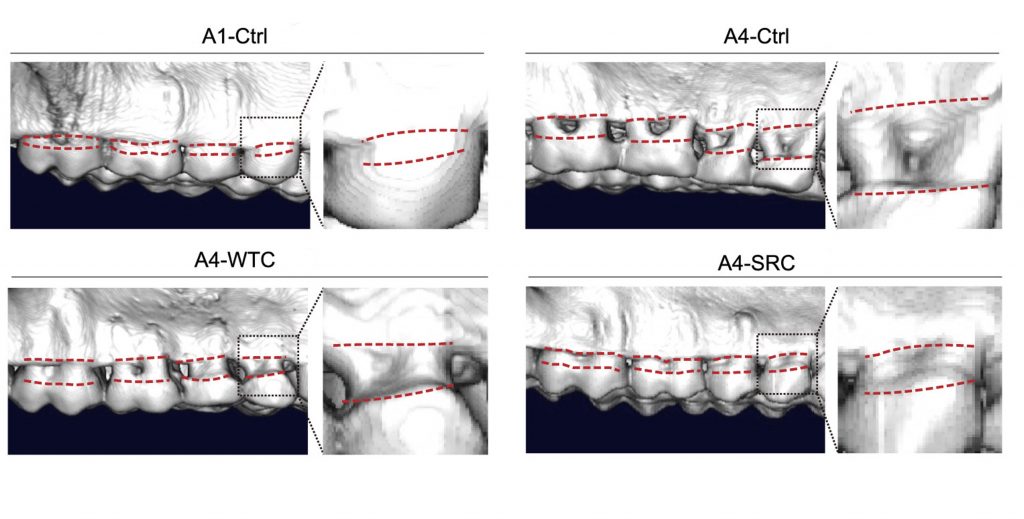
One common age-related disease is osteoporosis, characterized by brittle and weak bones that make patients more prone to fractures and deadly falls. Using an X-ray imaging technique called micro-CT, the researchers found evidence for the reversal of age-related bone loss. Namely, while the aged monkeys treated with saline exhibited dental bone loss, the aged monkeys treated with SRCs had teeth more similar to those of young monkeys.
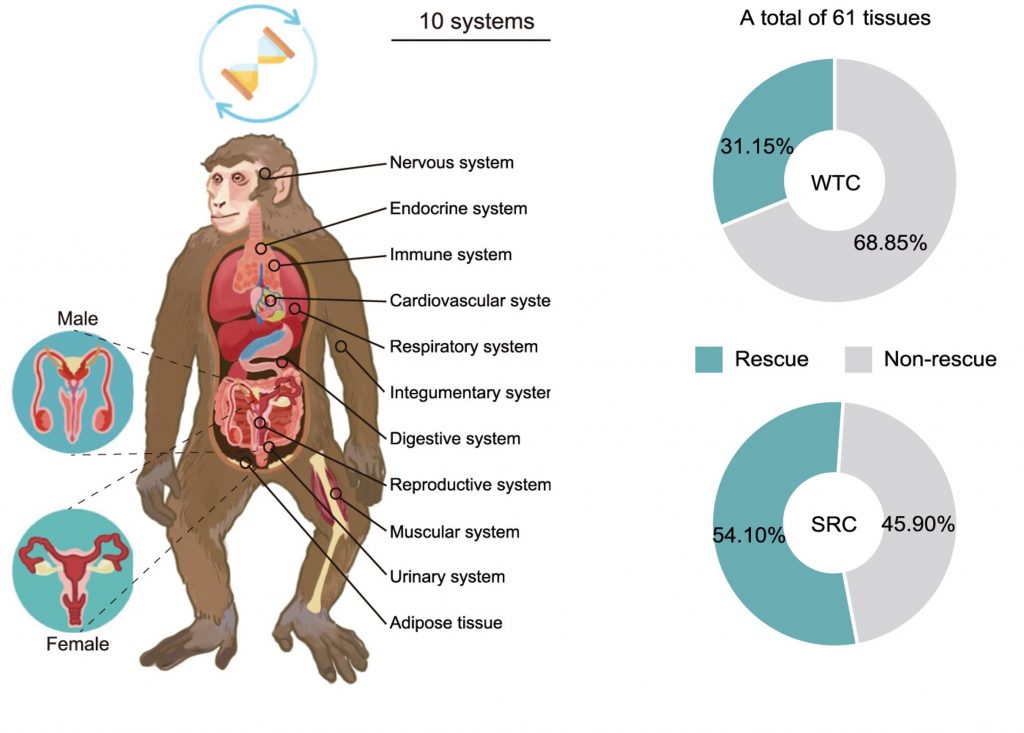
To conduct a body-wide assessment, the researchers measured the up- and down-regulation of genes from 10 systems and 61 tissues. Elevations and reductions in gene activation reflect the function (or dysfunction) of cells, tissues, and organ systems. With that said, SRC treatment was shown to rejuvenate over 50% of the tissues examined, with maximal rejuvenation achieved in areas like the hippocampus (the memory consolidation center of the brain), fallopian tubes, and colon. In contrast, the regular stem cells rejuvenated about 30% of the tissues examined.
Confirming some of the rejuvenating effects inferred by the gene experiments, the researchers also observed structural changes to various organs and tissues from aged monkeys treated with SRCs. For example, the vascularity of the lung and heart was improved while the thickening of the aorta was reduced. Neurons had longer projections and fewer proteins associated with Alzheimer’s disease (e.g., beta-amyloid and phosphorylated-tau), and the kidney and brain showed less mineralization [abnormal mineral (usually calcium) deposits].
SRCs Reduce Cellular Senescence and Inflammation
Modern scientists have begun to unravel the underlying causes of aging by identifying commonalities between age-related diseases at the cellular level. Two of the most prominent purported underlying causes of aging are chronic inflammation and senescent cells. With age, senescent cells accumulate throughout the body, promoting inflammation by secreting pro-inflammatory molecules.
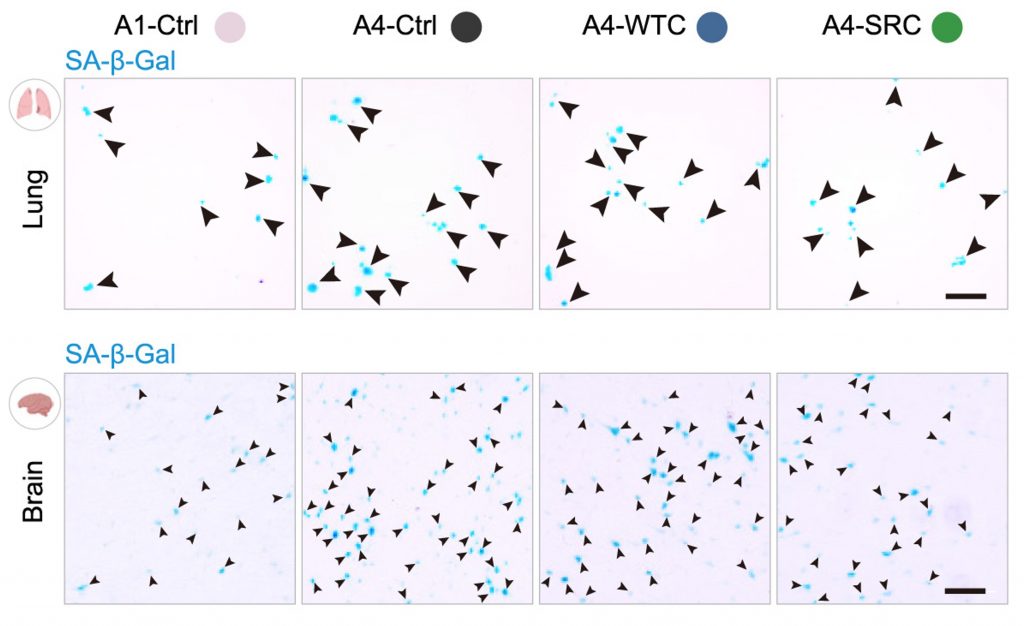
Eliminating senescent cells, which can be achieved through genetic manipulation or compounds called senolytics, ameliorates age-related diseases and even extends the lifespan of model organisms. Now, the Academy researchers demonstrate that SRCs reduce senescent cells, measured using a blue dye called SA-β-Gal, in multiple organs, including the brain, heart, and lungs. Along those lines, SRC treatment also reduced markers of inflammation and other underlying causes of aging, like DNA damage.
Stem Cells Make Sense
When it comes to combating degenerative aging, it makes sense that regenerative stem cells are a promising solution. In fact, one of the underlying causes of aging is stem cell exhaustion, whereby stem cells lose their regenerative capacity. While normal stem cells have anti-aging effects, as shown by the Chinese Academy of Sciences researchers, they are not protected against stressors like age-related inflammation. This explains why SRCs provide enhanced regenerative capacity (they withstand the harsh microenvironments induced by aging and cellular senescence).
As no serious safety concerns were raised during the study, it would seem that SRCs are well tolerated. However, the long-term effects of the SRC treatment will need further evaluation. The primary concern with injecting stem cells into the bloodstream is that they can trigger the spread of cancer almost anywhere in the body. Nevertheless, the SRCs possess tumor suppression properties, suggesting they may not induce tumor growth. If this ends up being true, we may soon see SRCs being tested in humans.