Onset through childhood and severe vision loss are characteristics of the degenerative disease known as Leber congenital amaurosis, which induces retina degeneration. Lately, mutations in NMNAT1, a crucial enzyme that helps facilitate the synthesis of nicotinamide adenine dinucleotide (NAD+), have been found in patients exhibiting Leber congenital amaurosis type 9. Thus, it is evident that a prominent feature of retinal degenerative disorders is the early-onset deficiency of NAD+ in the retina; however, we have yet to determine the biological pathways and cell types that are affected in patients with Leber congenital amaurosis.
In the journal eLife, an article published by Sasaki and colleagues from the Washington University School of Medicine, St. Louis, they found that decreasing the levels of cellular NNMAT1 gives rise to the selective loss of photoreceptors, important cells located in the eye’s retina that are in charge of turning light into signals that are delivered to the brain. Furthermore, investigators demonstrate that activation of SARM1, an enzyme that consumes NAD+ and executes nerve fiber degeneration, results from diminished levels of NMNAT1 and ultimately leads to vision loss and photoreceptor death. Hence, inhibiting SARM1 remains the primary function of NMNAT1 in photoreceptors. “Our identification of SARM1 as the executioner of photoreceptor death in this model of Leber congenital amaurosis type 9 opens up new therapeutic possibilities,” said the investigators in their study.
NMNAT1 serves crucial roles in various retinal functions. It has been shown that loss of retinal function in mice and extreme retinal dystrophy result from the decrease of NMNAT1 in the retina of developing mice. In addition, evidence suggests that the survival of mouse retinal progenitor cells increases upon the incorporation of NMNAT1. Surprisingly, while nearly every human body cell contains NMNAT1, patients with Leber congenital amaurosis fail to exhibit other medical complications other than degeneration of the retina. Nevertheless, very little is known about the exact functions of NAD+ and NMNAT1 in the retina.
The study presented by Sasaki and colleagues aimed to locate the cell types responsible for inducing retinal degeneration in Leber congenital amaurosis type 9. They found that loss of NMNAT1 caused rapid degeneration of photoreceptors and that diminishing levels of NMNAT1 in rod or cone cells adequately promote retinal degeneration and reduced retinal function. In addition, retinal degeneration can occur upon deletion of NMNAT1 by specific cell types in the photoreceptors of young mice. Hence, both mature and developing photoreceptors need NMNAT1 to survive and function properly.
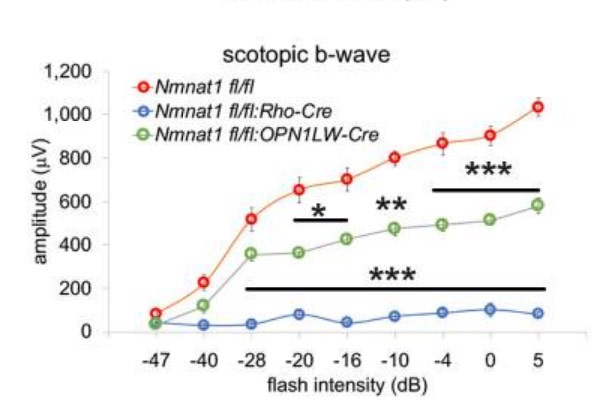

(Sasaki et al., 2020 | eLife) NMNAT1 is essential for the survival and function of rods and cones. Sasaki and colleagues observed a severe reduction in the rod photoreceptor function (scotopic) and in the cone-mediated photoresponses (photopic) when NMNAT1 was deleted from rods (Nmnat1fl/fl:Rho-Cre: Rho-Cre) and cones (Nmnat1fl/fl:OPN1LW-Cre: OPN1LW-Cre).
Investigators proceeded with demonstrating how retinal function can be ameliorated through viral-mediated gene replacement in photoreceptors. To achieve restoration of NMNAT1 in the retina, they utilized an adeno-associated virus (AAV), a naturally occurring, harmless virus that has been previously incorporated in gene therapy and aims to assist diseased cells in restoring structure and function. In the studied model of Leber congenital amaurosis type 9, Sasaki and colleagues demonstrated how preserving NMNAT1 in photoreceptors can somewhat recover retinal degeneration and boost retinal function.
The U.S. FDA recently authorized the use of AAV to serve as a therapeutic reagent for Leber congenital amaurosis type 2 and various retinal dystrophies that contain identified mutations. “Theoretically, LCA9 caused by the loss of NMNAT1 function is a reasonable target for AAV-mediated gene therapy,” said the investigators in their article. “Future studies will assess the efficacy of gene replacement after deletion of NMNAT1 to more closely mimic the human condition.”

(Sasaki et al., 2020 | eLife) AAV that replenishes NMNAT1 levels improves rod photoreceptor function in NMNAT1-deficient retinas. Sasaki and colleagues observed significant increases in rod photoreceptor function (scotopic) and small increases in cone photoreceptor function (photopic) from retinas treated with AAV replenishing NMNAT1 compared with retinas injected with control AAV (AAV-GFP).
Ultimately, Sasaki and colleagues wanted to develop a better understanding of how NMNAT1 facilitated the survival of photoreceptors. Results suggested that degeneration of photoreceptors was linked to reduced levels of NMNAT1. When the amount of NMNAT1 decreases, the SARM1 gene, which is known to promote axon degeneration in neurons, becomes activated. Furthermore, SARM1 mediates the degeneration of photoreceptors when NMNAT1 is not present.
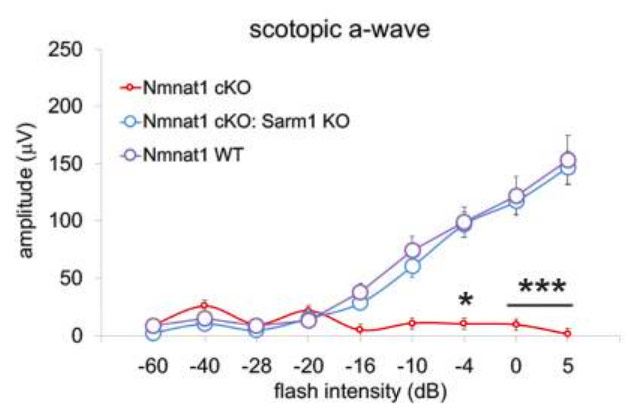


(Sasaki et al., 2020 | eLife) Depletion of SARM1 rescues the function of rods and cones in the NMNAT1-deficient retina. Sasaki and colleagues examined the functional consequences of NMNAT1 depletion in the presence or absence of SARM1 and found that loss of SARM1 prevented the severe loss of both rod (scotopic) and cone (photopic) photoreceptor responses due to NMNAT1 deficiency.
“This surprising result extends our understanding of both mechanisms causing retinal degeneration and the potential role of SARM1 in human disease,” said the investigators. “Since the SARM1 pathway is likely druggable, these findings provide a framework for developing new therapeutic strategies for treating patients with LCA9 and potentially other retinal disorders.”