When kidneys become damaged and can no longer filter blood properly, humans become susceptible to developing kidney disease. This has often been linked to malfunctional mitochondria, the cell’s power generator, and a reduction in the concentration of cellular nicotinamide adenine dinucleotide (NAD+), a vital compound that fuels functional mitochondria. However, there is no apparent connection between kidney disease and NAD+ deficiency.
In the journal Nephrology Dialysis Transplantation, Faivre and colleagues published a research article that showed the downregulation of a major NAD+ synthesis pathway in biopsies from the short-term phase (acute phase) of kidney transplant patients as well as in over 200 biopsies from long-term (chronic) kidney disease patients of various causes. Rodent models of acute kidney injury and chronic kidney disease were used to reproduce what was seen in human subjects. Researchers showed that kidney damage in rodent models of acute kidney injury could be attenuated by elevating NAD+ concentration through oral consumption of nicotinamide riboside (NR), a readily available potent precursor to NAD+. However, rodent models of chronic kidney disease failed to prevent chronic kidney disease progression after increasing NAD+ levels with NR supplementation. Researchers conclude that NR can be utilized to avert acute kidney injury in high-risk patients and prior to kidney donation or transplantation.
Acute kidney injury is a crucial risk factor that can further progress chronic kidney disease, a global health issue contributing to immense financial burdens, higher mortality, and lower quality of life. Acute kidney injury can arise from mitochondrial dysfunction and ultimately assist the progression of chronic kidney disease.
NAD+ is a vital compound located in all cells of the body and is fundamental to the formation and function of healthy mitochondria. De novo NAD+ synthesis is a cellular process that initiates the generation of NAD+ from tryptophan, an amino acid that serves as a critical protein building block and is commonly located in turkeys and other diverse foods. Another important pathway known as the salvage pathway can also utilize nicotinamide riboside (NR), nicotinamide, and nicotinic acid as precursors to synthesize NAD+.
While acute kidney injury is connected to deficient de novo NAD+ synthesis, researchers are still unaware of how chronic kidney disease progression and fibrogenesis are affected by changes in de novo NAD+ synthesis. Therefore, identifying the pathway utilized by the kidney for producing NAD+ is a critical therapeutic target that is currently being studied to combat kidney injury and disease. So, Faivre and colleagues used biopsies from kidney transplant patients and chronic disease patients of various causes to examine the concentration of key enzymes incorporated in NAD+ synthesis.
Investigators modeled acute kidney injury using transplanted human kidneys, and biopsies revealed stimulation of the salvage pathway and impairment of de novo NAD+ synthesis. After observing the salvage pathway and the NAD+ de novo synthesis pathway in chronic disease patients, biopsies showed preservation of the salvage pathway and impairment of the NAD+ de novo synthesis pathway, which corresponded to the stage of chronic kidney disease. “To our knowledge, we are the first to report alterations of NAD+ synthesis in all kidney compartments in human chronic kidney disease patients, with a severity proportional to the chronic kidney disease stage,” said the investigators in this article.
Multiple mouse models of chronic kidney disease and acute kidney injury supported the finding that de novo NAD+ synthesis was lowered. Investigators also evaluated the effects of NR supplementation in mouse models of chronic disease and acute kidney injury. Mouse models of acute kidney injury exhibited beneficial effects after supplementation with NR. However, NR supplementation was not successful in preventing the progression of chronic kidney disease.
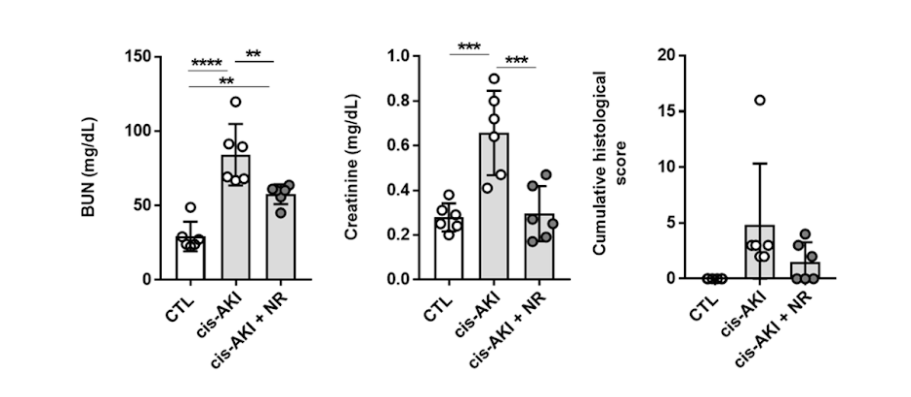
(Faivre et al., 2020 | Nephrology Dialysis Transplantation) Supplementation with NAD+ precursor NR alleviates acute kidney injury in mice. The investigators evaluated the effect of NR on markers of kidney injury that included blood urea nitrogen (BUN) and creatinine levels as well as scored the amount of damage (cumulative histological score) in a mouse model of acute kidney injury (AKI). These results show the NR treatment (cis-AKI + NR) significantly reduced the levels of blood urea nitrogen (left) and creatinine (center) as well as the cumulative histological score (right) compared to untreated mice with acute kidney injury (cis-AKI).
As a whole, the obtained results demonstrate that patients with acute kidney injury are more responsive than patients with chronic kidney disease to restoring NAD+ through NR supplementation. “We show in mouse models that NR is able to prevent acute kidney injury but does not attenuate chronic kidney disease progression,” said the scientists in their study.
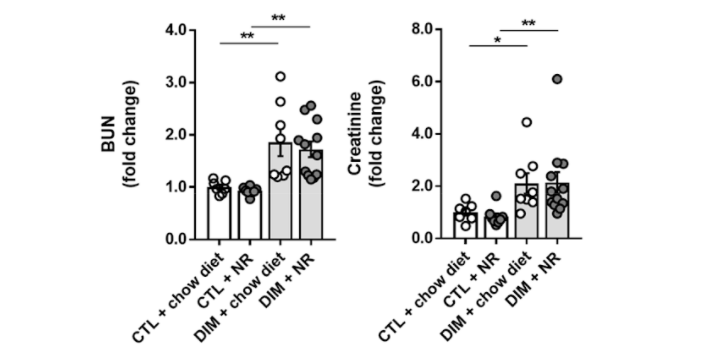
(Faivre et al., 2020 | Nephrology Dialysis Transplantation) Supplementation with NAD+ precursor NR does not alleviate chronic kidney damage in mice. The investigators evaluated the effect of NR on markers of kidney injury that included blood urea nitrogen (BUN) and creatinine levels in a mouse model of chronic kidney disease (DIM). These results show the NR treatment (DIM + NR) did not affect the levels of blood urea nitrogen (left) and creatinine (right) compared to untreated mice with chronic kidney injury (DIM + chow diet).
The authors of this study also propose that kidney recovery may ameliorate in kidney transplant recipients if NR is supplemented prior to transplantation. Given that patients with chronic kidney disease exhibited a lower response to NR supplementation, more experiments need to be conducted to elucidate alternate therapies for replenishing NAD+ and identify subpopulations that would make the most of these therapies.