Key Points:
- The lifespan-extending machinery of our cells converges on the powerhouse of the cell (mitochondria).
- Anti-aging interventions like physical exercise, dietary restriction, and metformin may be ineffective due to age-related mitochondrial decline.
- Anti-aging interventions that support mitochondrial health, like NAD+ boosters and rapamycin, bypass age-related mitochondrial decline.
For centuries, humanity has been captivated by the thought of turning back the aging clock. While a magic pill remains science fiction, researchers are uncovering fascinating insights into the biology of aging, and more importantly, how to influence it. A new review by Dr. Chaudhari and Dr. Ermolaeva from the Leibniz Institute on Aging in Germany parses age-influencing strategies based on which age demographic they benefit the most.
Some Anti-Aging Interventions are Not One-Size-Fits-All
In the pursuit of the fountain of youth, scientists have discovered ways to extend the lifespan of various model organisms, such as lab mice. These anti-aging methods include tried and true interventions like physical exercise and dietary restriction. Dietary restriction encompasses caloric restriction (eating fewer calories without malnourishment) and fasting (not eating for prolonged periods).
Other interventions include repurposed drugs like the anti-diabetes drug metformin and the immunosuppressant drug rapamycin. Genetic mutations also have powerful effects on longevity, such as mutations that block the insulin growth factor signaling (ISS) pathway. Additionally, mild stressors like heat stress and hypoxia (a lack of oxygen) are established interventions for prolonging the health and longevity of model organisms.
However, Dr. Chaudhari and Dr. Ermolaeva say,
“Despite significant progress in the development of longevity remedies in the past decade, the success of these interventions is highly dependent on physiological variables such as biological sex, genetic background, metabolism, and age of the treated individuals. As a result, there are significant differences in the presence and extent of interventional benefits.”
Lifespan-Extension Machinery Converges on Mitochondria
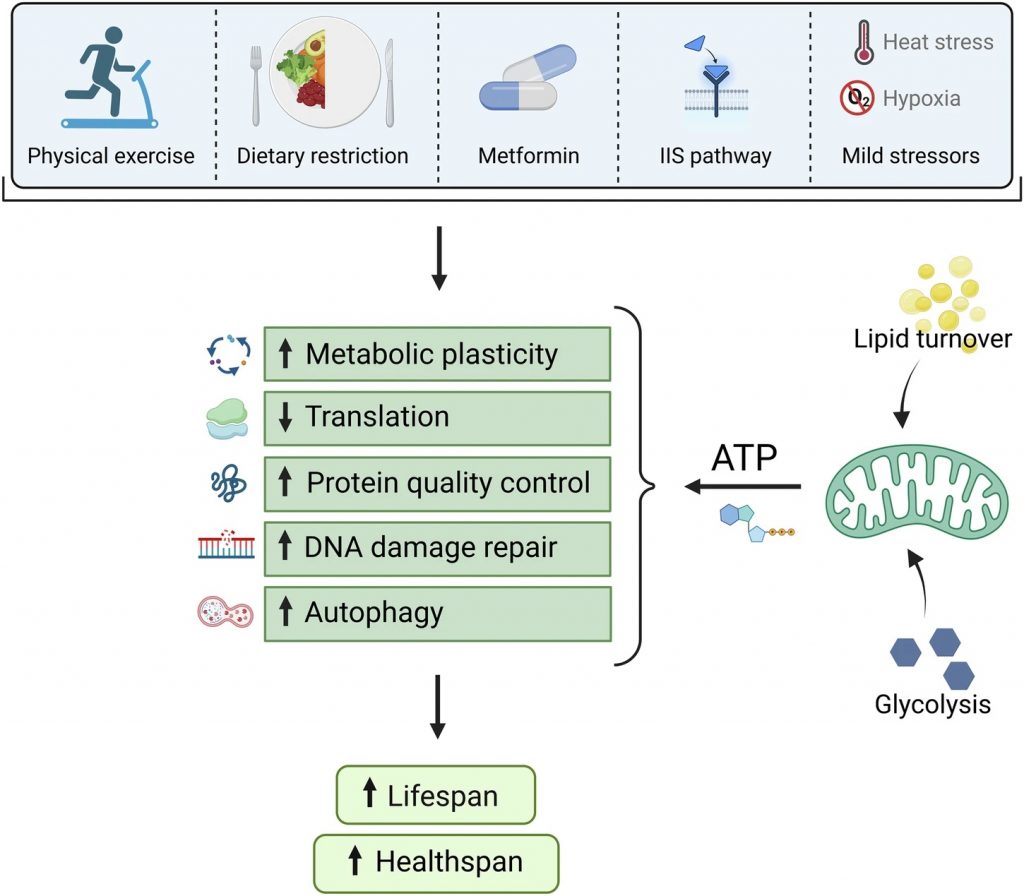
Chaudhari and Ermolaeva go on to list important longevity- and health-promoting cellular processes that scientists have uncovered over the years:
- Metabolic plasticity: The ability of cells to alternate between energy sources (e.g., from glucose to lipids).
- Translation: The process cells use to make proteins.
- Protein quality control: The processes that ensure proteins are folded into their correct three-dimensional structure.
- DNA damage repair: This process involves enzymes that consume NAD+, such as PARPs and sirtuins.
- Autophagy: The process our cells use to recycle damaged cellular material, such as misfolded proteins.
What most of these processes have in common is their intensive consumption of cellular energy, known as ATP (adenosine triphosphate). Since mitochondria are the primary source of ATP, all of these processes are dependent on mitochondrial health. In the case of metabolic plasticity, mitochondria are responsible for converting lipids and glucose through glycolysis into ATP.
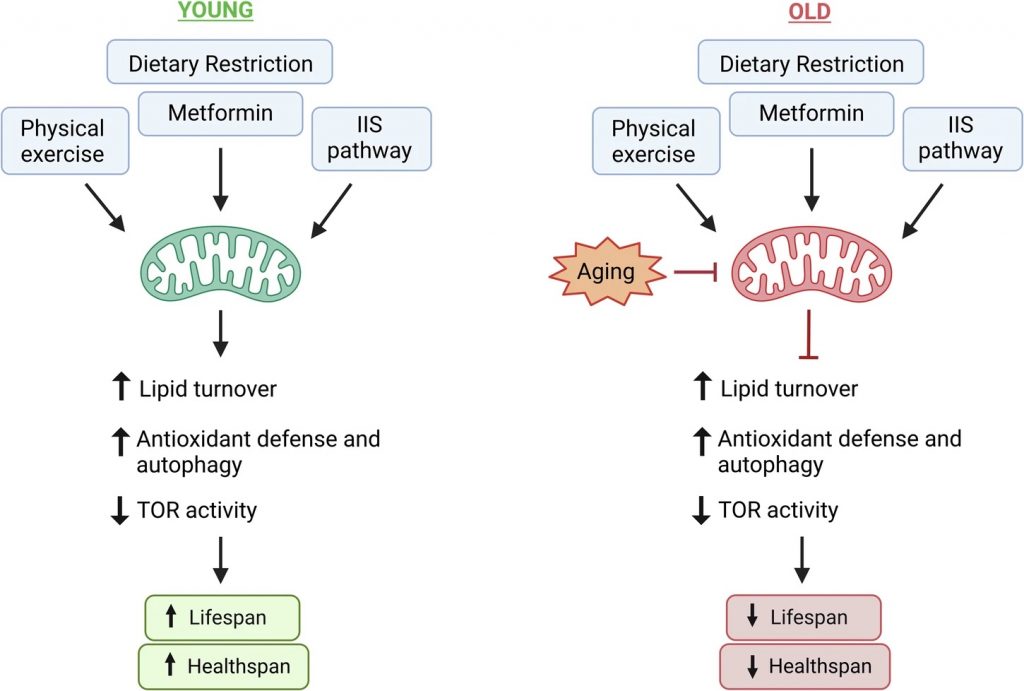
Anti-Aging Interventions Only Effective in Youth
Considering that some of the most effective anti-aging interventions rely on sufficient levels of ATP, Chaudhari and Ermolaeva argue that they may only be beneficial in youth when mitochondria are healthy. This idea stems from studies showing that, with age, mitochondria become dysfunctional. Thus, the interventions that rely on metabolic plasticity and sufficient levels of ATP from healthy functioning mitochondria would become less effective in older individuals. For example, Chaudhari, Ermolaeva, and others have shown that age-related mitochondrial dysfunction limits the lifespan benefits of dietary restriction and metformin.
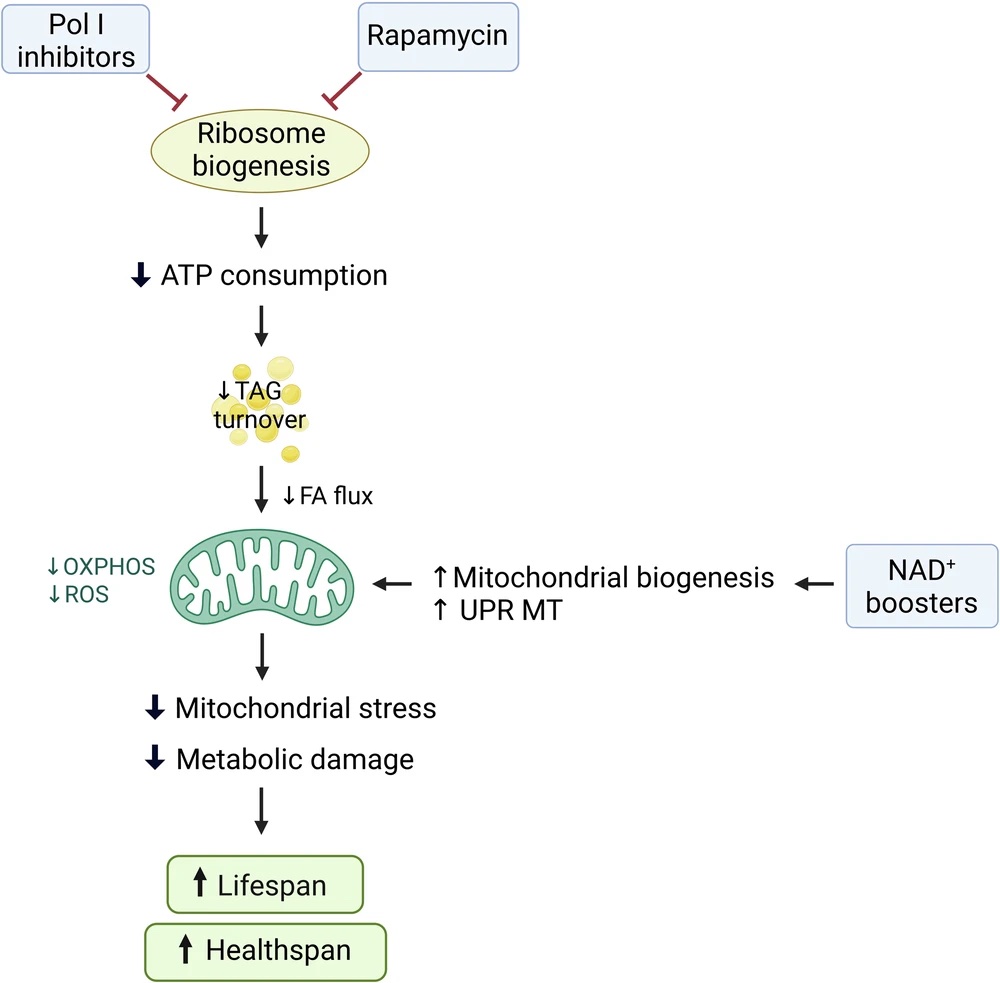
Anti-Aging Interventions Applicable to Older Individuals
On the premise that some anti-aging interventions benefit younger individuals but can be ineffective or detrimental to older individuals due to age-related decrements in ATP metabolism, Chaudhari and Ermolaeva identify interventions that do not rely on healthy mitochondria. Specifically, these interventions can be categorized as NAD+ (nicotinamide adenine dinucleotide) boosters, mTOR (mechanistic target of rapamycin) inhibitors, and polymerase I inhibitors.
NAD+ Boosters
NAD+ boosters are supplements, such as NR (nicotinamide riboside) and NMN (nicotinamide mononucleotide), that can help replenish NAD+ levels, which studies show decline with age. NAD+ doesn’t rely on, but helps improve mitochondrial health and boosts ATP production. It does so by promoting processes like mitochondrial biogenesis, the formation of new mitochondria. NAD+ is also involved in the mitochondrial unfolded protein response (UPR MT), which improves protein quality control. NAD+ also supports metabolic plasticity, as well as DNA repair via PARPs and sirtuins.
mTOR Inhibitors
The mTOR pathway is the master regulator of cell growth that modulates protein synthesis. It plays a crucial role in ribosome biogenesis, the synthesis of ribosomes, and the proteins responsible for translation. mTOR also blocks the beneficial process of autophagy. In older age, its overactivation can be draining, pushing cells to build and consume energy. This relentless activity contributes to cellular wear and tear. mTOR inhibitors like rapamycin apply the brakes, reducing translation and negating the need for excessive protein quality control. In promoting autophagy, mTOR inhibitors also promote mitophagy, which improves mitochondrial health.
Polymerase I Inhibitors
RNA polymerase I (Pol I) is an enzyme that activates genes for essential components of ribosomes. In this way, Pol I is vital for protein synthesis and translation. As such, inhibiting Pol I has similar effects to inhibiting mTOR in the context of translation and protein quality control.
A Caveat
As of today, there is a significant gap between these promising laboratory findings and large-scale, conclusive human studies. We do not yet have the definitive data to confirm that these compounds will provide the same remarkable longevity benefits in the vastly more complex human system. Questions of long-term safety, optimal dosage, and individual reactions in humans are still largely unanswered. So, while the map to a longer, healthier life is being drawn with ever-increasing detail, we must remember that we are still in the early days of exploration.