Key Points:
- The administration of a gene therapy to ensembles of neurons that store specific memories restores learning and memory in aged mice and those modeling Alzheimer’s disease.
- In both mouse models, the gene therapy also reversed aging-related neuronal hallmarks.
- The gene therapy reversed abnormal gene expression patterns in the neuronal ensembles.
Researchers and clinicians involved in regenerative medicine, a branch of medicine focused on regenerating damaged tissues and organs to restore normal function, have long sought to identify ways to counteract age-related cognitive decline. Recently, partial cellular reprogramming has emerged as a promising way to restore youthful function in tissues and organs, and Harvard’s David Sinclair uses this technique in experiments attempting to reverse aspects of aging.
Partial cellular reprogramming involves exposing mature cells briefly or cyclically to three proteins called Yamanaka factors, which are primarily expressed in early embryos, so the cells regain youthful characteristics. Whether this approach bears fruit in counteracting underlying cognitive decline during aging and in disease states like Alzheimer’s has remained unclear.
Now, as published in Neuron, Graff and colleagues from École Polytechnique Fédérale de Lausanne in Switzerland unveil data showing that aged mice and those modeling Alzheimer’s disease recover more youthful learning and memory capacities after undergoing partial cellular reprogramming via gene therapy targeting networks of neurons that store specific memories (known as engrams). The gene therapy also increased cell nucleus circularity, which has been shown to falter with age, in reprogrammed engram cells. Moreover, the gene therapy applied to engrams reversed aberrant gene expression patterns tied to synaptic plasticity, the ability of neuronal connections to strengthen or weaken over time in response to activity. These findings provide the first evidence that partial cellular reprogramming targeting engram neurons in the aged and Alzheimer’s disease-ridden brain can help to restore aspects of cell structure and gene expression and recover cognitive faculties.
“Taken together, our findings provide a proof of concept that targeting [partial cellular reprogramming] to engram cells in the aged and diseased brain can help to recover cognitive functions,” say Graff and colleagues in their publication.
Engrams Explained
Engrams are neural memory traces, ensembles of neurons that store specific memories. More specifically, they encompass a specific network of neurons that becomes activated, modified, and physically altered by a particular experience or learning event. Intriguingly, neurons within the engram are reactivated later to retrieve that memory.
Significant evidence suggests that dysfunctional engram networks, the neuronal ensembles responsible for memory storage and retrieval, underlie age-related cognitive decline. Similarly, engram impairments have been observed in mouse models of Alzheimer’s disease, particularly affecting memory retrieval. Due to this evidence, Graff and colleagues sought to target engrams with gene therapy using the promising technique of partial cellular reprogramming to restore aspects of cognition in aged mice and those modeling Alzheimer’s disease.
Partial Cellular Reprogramming Restores Cognitive Function in Aged Mice and Those Modeling Alzheimer’s Disease
To administer their partial cellular reprogramming gene therapy, Graff and colleagues performed cranial surgeries to deliver Yamanaka factors to neuronal ensembles activated during a fear-based learning task. The researchers performed this gene therapy procedure in older, nine- to 10-month-old mice (roughly equivalent to age 40 in humans) and young, two- to three-month-old mice (roughly equivalent to age 20 in humans).
Graff and colleagues utilized a gene therapy system that turns on the expression of Yamanaka factors in engrams when a specific molecule is removed from drinking water. With their gene therapy technique, Graff and colleagues synchronized the expression of Yamanaka factors in the brain to the learning phase of a fear-based conditioning protocol. In the conditioning protocol, the mice associated a foot shock with a particular environmental setting and would freeze from fear when placed in this environment if they had learned to associate it with the foot shock. When the fear-based conditioning protocol ended, the mice were reintroduced to the molecule that turns the gene therapy off, thus ceasing the expression of Yamanaka factors and the wave of partial cellular reprogramming.
To determine whether partial cellular reprogramming gene therapy rescues short-term learning and memory capabilities, Graff and colleagues first administered the gene therapy to a region of the brain called the dentate gyrus (DG) of the hippocampus. This brain region is crucially involved in forming new memories, encoding memories for retention, and remembering where objects are spatially in a given environment.
To test short-term learning and memory capabilities, Graff and colleagues first exposed aged mice that did not undergo the gene therapy to the fear-based foot shock conditioning. Compared to young mice, the older mice exhibited less freezing when placed in the fear-inducing, foot-shock-associated environment, suggesting age-related impairments in fear-based memory.
Graff and colleagues then sought to test short-term learning and memory capabilities in old mice that underwent partial cellular reprogramming gene therapy in the DG of the hippocampus. Thus, they performed the same experiment in old mice that underwent the gene therapy. Interestingly, the fear-based memory in these mice was rescued, similar to that exhibited by young mice. These findings suggest that engram-targeting partial cellular reprogramming gene therapy can counteract age-related deficits in short-term fear-based associative learning and memory.
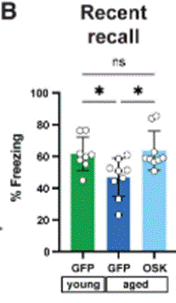
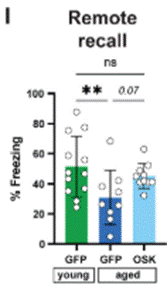
Graff and colleagues then sought to assess the effect of partial cellular reprogramming gene therapy on more durable forms of memory. To this end, they targeted engrams in a brain region called the medial prefrontal cortex (mPFC), which have been shown to be relevant for the recall of more remote memories not recently acquired or consolidated. As such, the mPFC is involved in the storage and retrieval of remote memories—learned experiences from the past, acquired over time ranges of weeks to years.
As with the previous experiment involving the DG of the hippocampus, engrams in the mPFC of aged mice were targeted with partial cellular reprogramming gene therapy during fear conditioning. The older mice were then tested for remote memories two weeks later. Aged mice that did not receive the gene therapy showed reduced freezing behaviors compared to young mice, suggesting impaired remote memory formation and retention. However, aged mice that received the gene therapy targeting engrams in the mPFC did not exhibit this impairment in remote memory. These results suggest that the partial cellular reprogramming gene therapy can rescue more remote memory formation and recall in older mice.
Because a shift in cellular nuclei from smooth, spherical shapes to irregular, elongated shapes is a characteristic of cellular aging, Graff and colleagues sought to determine whether engram cells of aged mice that received the gene therapy displayed this hallmark of cellular aging. When they examined the cellular nuclei of these engram cells, they found the nuclei had a more circular structure. Thus, these findings suggest that, indeed, partial cellular reprogramming gene therapy restores this cellular hallmark of aging in engram neurons. Through its rejuvenating effects on engram cell nuclei shapes, along with potential positive effects on other cellular structures, partial cellular reprogramming may improve engram neuronal function.
Graff and colleagues then sought to find whether partial cellular reprogramming gene therapy targeting engrams in the context of neurodegenerative disorders, such as Alzheimer’s disease, has a similar effect to that observed in older mice. Intriguingly, in sets of experiments similar to those performed in aged mice, which targeted either the DG of the hippocampus or the mPFC, Graff and colleagues found that targeting engrams with partial cellular reprogramming can rescue short-term learning and memory as well as the formation and retrieval of more remote memories in mice modeling Alzheimer’s disease. These findings provide evidence that certain aspects of learning and memory can be rescued in a neurodegenerative condition with the use of partial cellular reprogramming gene therapy.
Next, Graff and colleagues analyzed data reflecting changes to gene activation patterns (transcriptional data) from the mice modeling Alzheimer’s disease that underwent the engram-targeting gene therapy. In doing so, they sought to uncover whether partial cellular reprogramming gene therapy rescues aspects of gene activity related to neural function in Alzheimer’s disease. Interestingly, they found that a significant fraction of genes with altered expression in the engrams of mice modeling Alzheimer’s disease showed restoration toward normal expression levels following the gene therapy. Some of the genes showing such restoration of expression levels were linked to neural connection organization. Taken together, these transcriptional data suggest that partial cellular reprogramming, to some degree, corrects abnormal gene activation patterns associated with the organization of neural connections in mice modeling Alzheimer’s disease.
An Optimistic Prediction of Partial Cellular Reprogramming Available for Humans In 15 to 20 Years
Previous research using partial cellular reprogramming in mice has shown that the procedure can successfully extend lifespan and also promote the regeneration of pancreatic, liver, and muscle tissue. Furthermore, this technique has shown efficacy in restoring vision in a mouse model of blindness. As far as cognition goes, partial cellular reprogramming approaches, beginning during young ages, have been observed to preserve cognitive abilities during aging and disease. However, Graff and colleagues’ study provides some of the first evidence that engram-targeting partial cellular reprogramming can rescue learning and memory functions after the onset of cognitive decline, as shown in older mice and those modeling Alzheimer’s disease.
Moreover, if this gene therapy technique works in the age-related neurodegenerative condition of Alzheimer’s disease, the possibility looms that it could also work in other neurodegenerative conditions, such as Parkinson’s disease. Accordingly, future research should test engram-targeting partial cellular reprogramming in other neurodegenerative disorders.
Whether partial cellular reprogramming will work in humans awaits human trial testing. Importantly, Harvard’s David Sinclair and his colleagues have received the FDA’s approval to start human trial testing using a similar gene therapy technique in patients with two eye conditions that lead to loss of vision. The overarching goal of Sinclair and his colleagues’ gene therapy is to restore vision to some degree in these patients. If Sinclair and colleagues’ human trials are successful, this could open the door to testing similar gene therapies that target engram cell rejuvenation to improve cognition in aging patients and those who have age-related neurodegenerative conditions, such as Alzheimer’s disease.
In essence, if Sinclair and colleagues’ human trial yields promising data for the efficacy of partial cellular reprogramming, this technique could eventually serve as a way to restore vision and rejuvenate multiple organs, like the brain. Thus, perhaps an optimistic prediction for the availability of this type of gene therapy for human use to restore cognition during aging or with a neurodegenerative condition would be in the next 15 to 20 years.