Highlights
- A DNA variant in the gene BPIFB4 is shared amongst long-living individuals.
- This longevity linked DNA variant eliminates senescent cells and rejuvenates NAD+ levels in mice.
- Long-living individuals, especially those with the longevity variant in BPIFB4, have higher NAD+ levels than elderly adults.
What makes us live longer? The food we eat, the amount of exercise we get, and the quality of the air we breathe all contribute. In addition to these somewhat controllable factors, we know that our genes contribute to aging as well – but how?
As reported in Cell Death & Disease, Ciaglia and colleagues from the University of Salerno in Italy showed that transferring a variant of a gene called BPIFB4 to old mice reduces the spread of senescent cells. This minor change in DNA sequence stops the senescent cells from activating CD38, an enzyme that breaks down NAD+. Interestingly, people who live longer than average and have this version of the BPIFB4 gene have high levels of NAD+.
The Longevity-Associated Variant of BPIFB4
By today’s standards, most of us won’t live beyond the age of 85. Only some of us may become “long-lived individuals” and live over the age of 95. So, why do only some of us become long-living individuals? Scientists may have found out, as they have discovered a gene variant that many long-living individuals share, the longevity-associated variant (LAV) of BPIFB4 (LAV-BPIFB4).
We all have a version of BPIFB4, which helps fight off pathogens, however, LAV-BPIFB4 has a genetic mutation that allows it to do more. While normal BPIFB4 is trapped within the nucleus of cells, LAV-BPIFB4 can escape to the cytosol. By virtue of its location in the cell, LAV-BPIFB4 can interact with proteins that normal BPIFB4 cannot. In this way, LAV-BPIFB4, or the “longevity gene” can initiate processes that lead to increased lifespan.
Longevity Gene Variant Eliminates Senescent Cells to Restore NAD+
As we age, our organ systems decline, making them less efficient. Part of the reason for this decline is the buildup of senescent cells. Senescent cells are growth-arrested cells that secrete numerous molecules, including those that transform other cells into senescent cells. Unlike senescent cells trapped in a given organ, senescent immune cells can travel through the bloodstream, allowing them to transform any cell in the body.
To study the effect of LAV-BPIFB4 on senescent cells, Ciaglia and colleagues injected an adeno-associated virus (AAV) carrying LAV-BPIFB4 into old mice. They found that introducing LAV-BPIFB4 into the bloodstream of the old mice decreased not only the number of senescent immune cells but also the number of senescent cells in the aorta. This suggested that a lack of mobile senescent immune cells decreased the number of transformed aorta cells.
Senescent cells also secrete molecules that activate CD38 – an enzyme that breaks down NAD+. Therefore, by eliminating senescent cells, CD38 cannot be activated to break down NAD+. To test this, the Italian researchers measured the NAD+ levels of old mice injected with LAV-BPIFB4. They found that the longevity gene brought NAD+ levels back to the same level as young mice, as predicted.
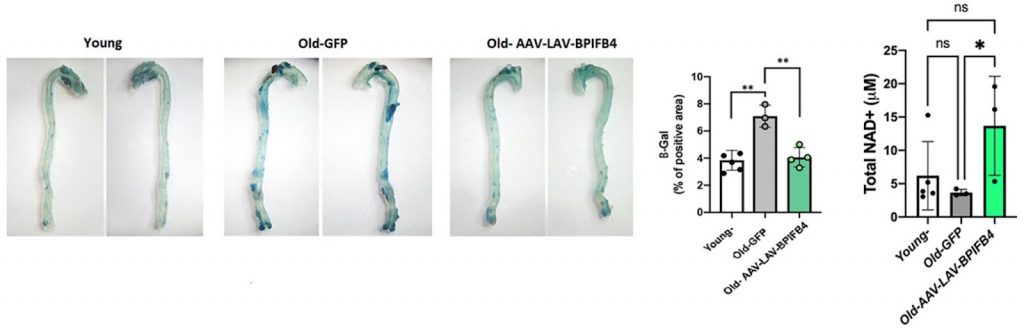
(Ciaglia et al., 2022 | Cell Death and Disease) LAV-BPIFB4 Reduces Senescence and Raises NAD+ in Mice. (A) Mouse aortas stained for a marker of senescence (blue, β-Gal). LAV-BPIFB4 was introduced into mice by injecting an adeno-associated viral vector (AAV-LAV-BPIFB4), whereby LAV-BPIFB4 replaced the infection-causing genes. This decreased senescence in the aortas of old mice. (B) NAD+ levels in old mice treated with LAV-BPIFB4 are restored to those of young mice.
Long-living Individuals have Higher Levels of NAD+ than Elderly Individuals
To validate what they found in mice, Ciaglia and colleagues measured the NAD+ levels of middle-aged adults, elderly adults, and long-living individuals. They found that long-living individuals had higher NAD+ levels than elderly adults. Furthermore, of the long-living individuals, those endowed with LAV-BPIFB4 had the highest levels of NAD+, demonstrating that LAV-BPIFB4 likely increases NAD+ levels in humans.
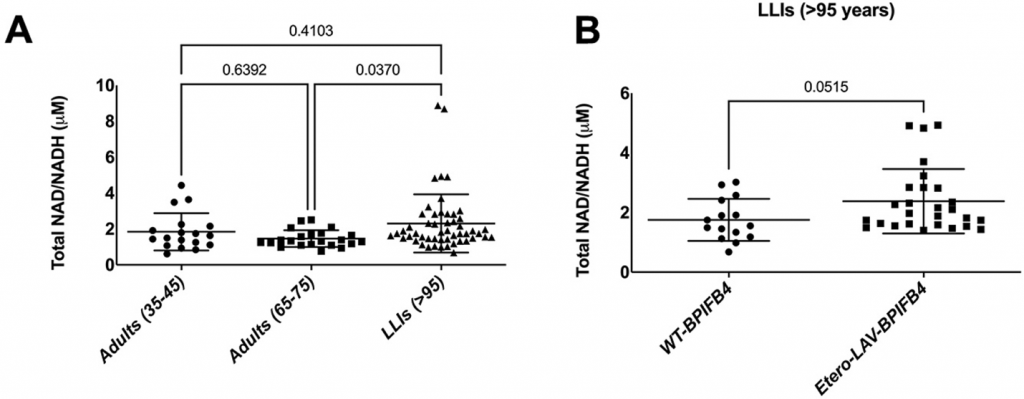
(Ciaglia et al., 2022 | Cell Death and Disease) High NAD+ Levels in Long-Living Individuals. (A) NAD+ levels are higher in long-living individuals (LLIs) compared to elderly adults. (B) LLIs with LAV-BPIFB4 (Etero-LAV-BPIFB4) tend to have higher NAD+ levels than LLIs without LAV-BPIFB4.
Is NAD+ the Key to Longevity?
The decline of NAD+ is associated with almost all age-related diseases. Ciaglia and colleagues have demonstrated that, by eliminating senescent cells, LAV-BPIFB4 raises NAD+ levels. Also, long-lived individuals, especially those with LAV-BPIFB4 have higher levels of NAD+ than elderly individuals. Is this human evidence for NAD+ increasing lifespan? Considering the difficulty in measuring the effect of NAD+ on lifespan in humans, this may be the closest we have.
Doomed to Live an Average Lifespan?
The results of Ciaglia colleagues show that LAV-BPIFB4 could counteract aging. But if we were not born with LAV-BPIFB4, are we doomed to live an average lifespan? Maybe not, since there are already FDA approved gene replacement therapies available. The technology is there, LAV-BPI B4 just needs to be tested in clinical trials, although more pre-clinical studies are likely needed.
Also, having high NAD+ levels in old age is not solely dependent on having LAV-BPIFB4. Numerous studies have shown that supplementation with NAD+ precursors like nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) boost NAD+ levels in old age. Whether or not this increases lifespan is still unknown, but the findings of Ciaglia and colleagues are encouraging, suggesting that sustained NAD+ levels throughout a lifespan can mitigate organ failure and death.