Key Points:
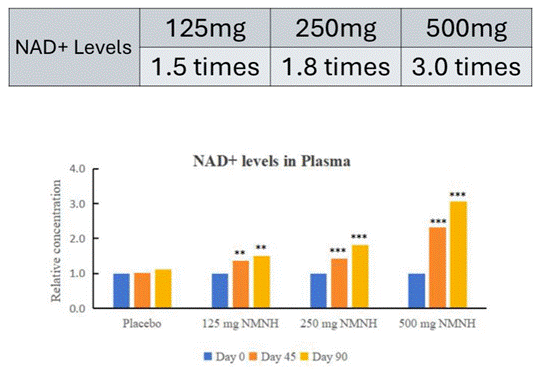
- NMNH, taken for 90 days at 500 mg doses, approximately tripled circulating levels of NAD+ in healthy adult participants.
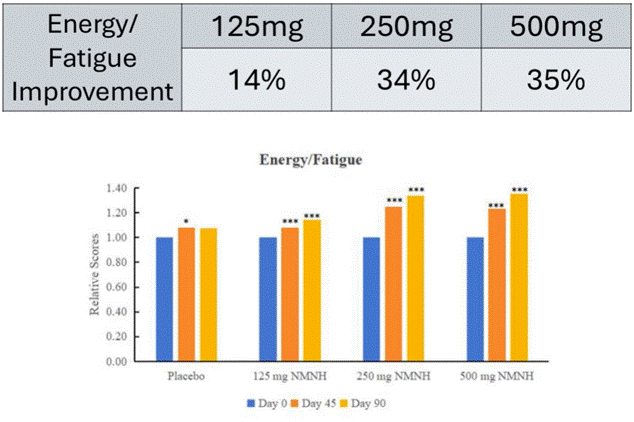
- Participants who took NMNH reported improvements in subjective measurements of emotional well-being and energy levels.
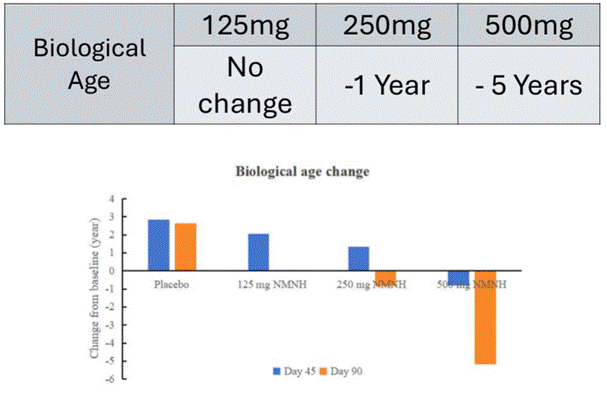
- According to the trial, NMNH was associated with a five-year reduction in biological age, but the means researchers used to assess biological age remain unknown.
As presented in a Modern Healthspan YouTube segment, longevity aficionado and host Richard Morgan shared details regarding data from the first human trial involving reduced nicotinamide mononucleotide (NMNH). According to his podcast segment, Morgan received a file from the company that sponsored the study, which contained the study’s data. Also, an alternative source, a recent press release available online, provides broad data similar to that presented in Morgan’s presentation, albeit without in-depth details.
Background on NMNH
NMNH (also known as dihydronicotinamide mononucleotide) is a precursor to nicotinamide adenine dinucleotide (NAD+) with a molecular structure similar to nicotinamide mononucleotide (NMN). The key difference between NMNH and NMN is that NMNH has an added hydrogen atom with two extra electrons. Furthermore, research with mice has suggested that NMNH raises NAD+ more potently than NMN, for example, roughly doubling NAD+ levels in tissues like the liver compared to NMN supplementation. Thus, figuring out whether NMNH can more potently raise NAD+ and confer potential physiological benefits more effectively than NMN has awaited human testing.
The Latest Results from the First-of-Its-Kind NMN Human Trial
The stated objectives of the NMNH human trial were to assess the safety and tolerability of NMNH, to measure NAD+ levels in the blood, and to look at broader outcomes, like quality of life and biological age (an age assessment based on how well cells and tissues function). For this purpose, 80 healthy adults aged 40 to 65 were split into four groups: one that received a placebo and three others that received 125 mg, 250 mg, or 500 mg of NMNH daily for 90 days.
As for what the unpublished and non-peer-reviewed results of the trial say, Morgan reported that NMNH approximately tripled circulating levels of NAD+ in a dose-dependent manner. In other words, over the course of 90 days of supplementation, at lower doses of 125 mg per day, NAD+ levels increased 1.5 times; at 250 mg daily doses, NAD+ increased 1.8 times; and with the 500 mg daily doses, NAD+ increased about threefold, compared to the placebo group.
A key drawback to these results is that researchers who performed the study did not include a group administered NMN for comparison. However, a search of the literature suggests that NMN supplementation can nearly double circulating NAD+, with the degree of increased NAD+ depending on the dose, duration, and individual taking the supplement. Thus, comparing the approximately threefold increase in circulating NAD+ from NMNH to NMN supplementation suggests NMNH may more potently increase circulating NAD+. All the same, a direct comparison that includes a group taking NMN as well as groups taking different doses of NMNH in the same study is necessary for confirmation.
More data showed that study participants reported improvements in how they felt day to day. For instance, the data showed that those who took 125 mg of NMNH daily showed a 14% improvement in energy levels, a 34% improvement at 250 mg doses, and a 35% improvement at 500 mg doses. Additionally, participants reported a 13% increase in emotional well-being at the 125 mg dose, a 26% improvement in emotional well-being at the 250 mg dose, and a 31% improvement at the 500 mg dose. Thus, NMNH improved parameters related to how participants felt day to day.
These results were obtained through the administration of the SF-36 to participants following taking NMNH for 90 days. The SF-36 is a short-form health survey that uses questions to assess dimensions of quality of life and mental health status. Moreover, the SF-36 is a standard, validated tool used widely in clinical research.
The two subscales of the SF-36 that registered significant findings after taking NMNH were energy levels as well as emotional well-being. For comparison, the SF-36 has been applied in a human trial where participants took NMN, and NMN conferred significantly improved scores for some parameters of the SF-36 questionnaire. However, the interpretation of the SF-36 scores for the NMNH human trial for comparison is tricky, because the results were reported as percentages. Normally, SF-36 scores are not reported as percentages but rather, absolute point changes on a zero to 100 point scale with clear comparisons showing differences between groups. As such, the way the SF-36 scores were reported in the NMNH human trial makes judging the clinical magnitude of NMNH’s effects on energy levels and emotional well-being very difficult.
Based on the incompleteness and obscurity of the SF-36 data reported from the NMNH human trial, the correct interpretation of the data is that the participants reported feeling better, more energetic, and with better emotional well-being. However, the size and robustness of this effect remain uncertain, and whether NMNH improves SF-36 scores better than NMN remains virtually impossible to assess.
The summary also reported a reduction in biological age of five years. Biological age is a measurement of age that reflects the body’s physiological condition and cellular health, indicating how well the body functions and ages, rather than the number of years one has lived.
The means that researchers used to calculate biological age were not disclosed in the report Morgan obtained. Biological age is not an agreed-upon scientific metric, and without knowing what method researchers used to calculate biological age, there is little that can be drawn from the biological age data associated with taking NMNH.
Importantly, safety data regarding NMNH are reassuring. Over 90 days, taking NMNH was not associated with any significant changes in liver markers, kidney markers, lipids, or urine analysis markers. This suggests that NMNH is well-tolerated over the short term in healthy adults; however, the NMNH human trial was a small study for a short, 90-day duration, so researchers cannot rule out adverse effects from taking NMNH over longer durations.
The Interesting NMNH Early Human Data Needs Replication
Collectively, the data from the first NMNH human trial suggest that NMNH appears to raise NAD+ in humans significantly, is well-tolerated over 90 days, and participants who took NMNH report improvements in subjective energy and mood. At the same time, the results are still unpublished, reported only by the sponsor company, and they lack key details needed to judge clinical significance.
The NMNH human trial data could be considered interesting, though, since it is so far the only NMNH human trial that has been completed. Also, the company that sponsored the study may have a conflict of interest, since it sells NMNH, but if the company publishes the results with raw data in a peer-reviewed journal, other research groups can run similar studies to find whether these data are reproducible to help verify their accuracy.