Key Points:
- Obese mice have a dysregulated gut microbiome (bacteria and other microbes in the intestines) that is restored by eating within an 8-hour window.
- This time-restricted feeding improves sugar and fat metabolism, preventing weight gain.
- Time-restricted feeding also leads to the gut microbiome producing more GLP-1, a molecule that improves sugar metabolism.
Our bodies undergo physical and mental changes along a 24-hour cycle, known as circadian rhythms. Research has shown that our gut microbiome follows similar rhythms. Now, a new study shows that perturbing these gut microbiome rhythms could contribute to obesity, suggesting that it is not what we eat, but when we eat that could cause us to gain weight, a problem faced by many aging adults.
The study, from scientists at the University of California, San Diego, published in Cell Reports focused on obese mice fed within an 8-hour window (time-restricted feeding) versus obese mice allowed to eat over 24 hours. Dantas Machado and colleagues found that mice with time-restricted feeding patterns had improved gut microbiome circadian rhythms. Time-restricted feeding also improved body weight and blood sugar levels. Additionally, GLP-1, a protein partially responsible for insulin secretion, was increased during feeding times in mice following time-restricted feeding, and bile acid – which is important for metabolizing fat – was also increased, suggesting these molecules contribute to improved metabolism and body weight.
“What we’ve learned is that cyclical changes in the gut microbiome are quite important for health since they help with the circadian clock, and with that the regulation and control of glucose, cholesterol and fatty acids – and overall metabolic health,” senior author Amir Zarrinpar said in a press release.
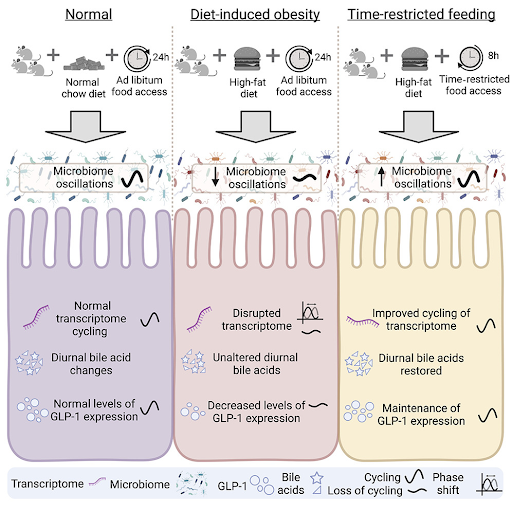
Time-Restricted Feeding Restores Changes Caused by High-Fat Diet
Mice were fed a high-fat diet with or without time restrictions and compared to mice given a normal diet. The mice on a time-restricted diet displayed improved body weight and blood sugar levels, despite having a similar caloric intake as the mice given unlimited access to high-fat food. Gut microbiome samples, which were collected every 4 hours over a 24-hour period, showed that the composition and cyclical changes of the microbiome were disrupted by the high-fat diet. However, time-restricted feeding restored the cyclical variations – normal daily changes in the gut microbiome – to levels similar to mice fed a normal diet.
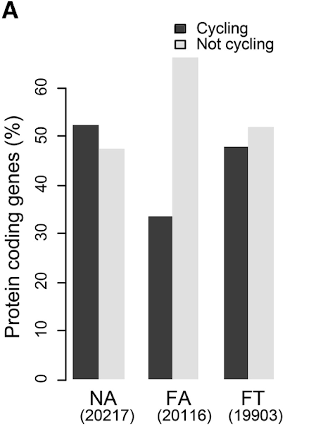
Dantas Machado and colleagues also found that the number of cycling activated genes (the transcriptome) within the small intestine was decreased in mice fed a high-fat diet compared to mice on a normal diet. However, the cycling of these genes was partially restored by time-restricted feeding. These genes were related to fat metabolism, autophagy (cellular recycling), and circadian rhythms, indicating that the prevention of obesity with time-restricted feeding may be due to the maintenance of microbiome gene activation dynamics.
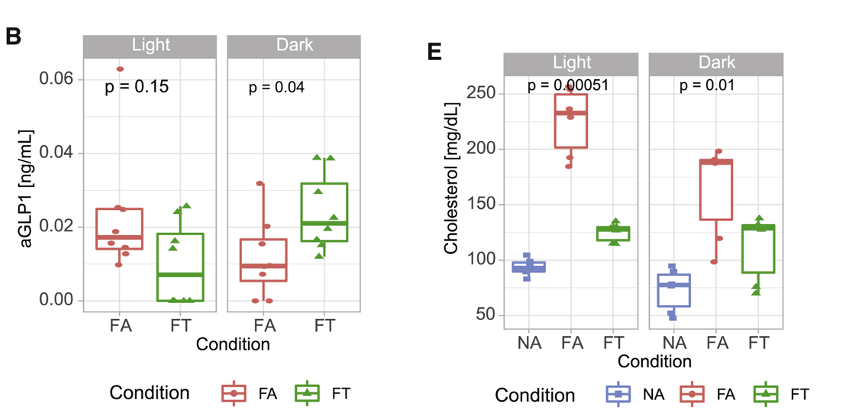
Time-restricted feeding was also shown to improve cells’ ability to respond to insulin (which regulates sugar uptake into cells) and the way in which fat is stored in the body in mice given a high-fat diet, which may be due to its effects on GLP-1 – a protein that stimulates insulin secretion leading to better metabolic function. Time-restricted feeding increased the levels of a GLP-1 precursor and maintained its normal cyclical nature, which is dampened when mice are given unlimited access to a high-fat diet. Bile acid signaling – important for fat absorption, glucose (sugar) metabolism, and energy balance – was also dysregulated with a high-fat diet and restored with time-restricted feeding.
The gut microbiome, particularly in the section of the small intestine examined in this study (ileum), is known to be a major contributor to metabolic signaling regulation. Dantas Machado and colleagues show how it is dysregulated by a high-fat diet. Yet, this dysregulation is alleviated with time-restricted feeding patterns, perhaps indicating that when you eat may be more important than what you eat, at least when it comes to metabolizing food efficiently and looking lean.
Is GLP-1 the Key to Time-Restricted Feeding’s Benefits?
Some of the effects seen on metabolism in the mice with time-restricted feeding patterns may be due to the increased GLP-1 seen during their feeding times, leading to increased metabolism, better nutrient utilization, improved body weight, and improved blood sugar, all of which would lead to healthier aging. If that is the case, GLP-1 supplementation may be a viable option for those who don’t want to quit their midnight snacks. Many anti-diabetes medications work through GLP-1, and many common household foods, including cinnamon and curcumin, have been shown to activate one’s own GLP-1. Additionally, several of the available GLP-1 supplements on the market contain herbs that are intended to activate one’s own GLP-1, leading to weight loss and increased metabolism, whether they actually work or not has not been proven.