Key Points:
- The shortening of telomeres induces senescence — aged, non-proliferating (senescent) cells that contribute to age-related disease.
- Restoring telomere length in aged human cells reduces the spread of senescence.
- Telomere restoration therapy extends the lifespan of aged mice by 20%.
Our telomeres shorten as we age, causing our DNA repair systems to malfunction. In response, affected cells convert to a senescent state, secreting molecules known as the senescence-associated secretory phenotype (SASP). These SASP factors include molecules that induce senescence in surrounding cells, which can lead to age-related disease. Recently, scientists have been trying to find ways to reduce senescent cells and their SASP factors to slow, prevent, or reverse the aging process.
Now, researchers from the Houston Methodists Research Institute in Texas report in the European Heart Journal that they can reduce the harms of senescence and prolong the lifespan of mice by extending telomere length. Utilizing an enzyme called telomerase to lengthen the telomeres of prematurely aged human cells, Mojiri and colleagues reduce senescence, allowing for an increase in blood vessel cell proliferation. Furthermore, it’s shown that the lifespan of prematurely aged mice is prolonged with telomerase gene therapy.
Telomere Lengthening Subdues Senescence in Human Cells
Mojiri and colleagues examined the effects of telomerase on cells from patients with Hutchinson-Gilford progeria syndrome, an accelerated aging syndrome associated with premature vascular aging. Impairment of endothelial cells (ECs) — the cells that line our blood vessels — is a primary cause of vascular aging. As would be expected from aged cells, the investigators showed that progeria ECs have increased levels of senescent cells and SASP factors, among other impairments.
By treating the progeria ECs with telomerase, the Houston researchers observed an increase in telomere length and improved EC function, including increased proliferation. Moreover, the telomerase therapy reduced senescence (senescent cells and SASP factors). Furthermore, the muscle cells that allow our blood vessels to dilate and constrict (vascular smooth muscle cells) could proliferate again due to reduced SASP factors from the ECs. Thus, telomerase therapy alleviates the defects associated with vascular aging by preventing the spread of senescence.
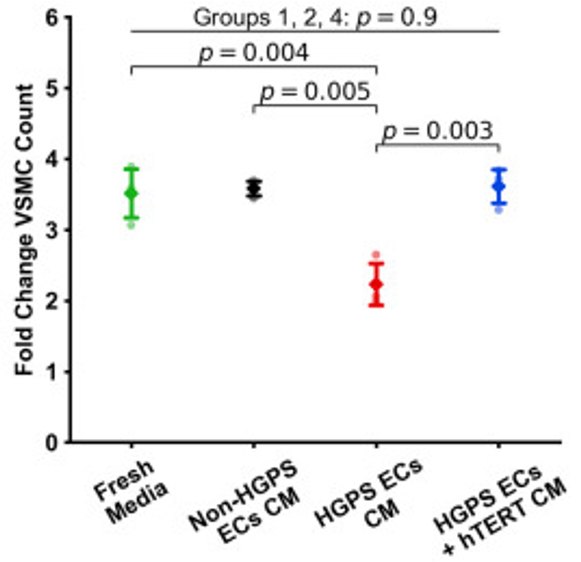
Telomere Lengthening Prolongs Mouse Lifespan
Mojiri and colleagues next tested the effect of telomerase therapy on a mouse model for progeria (age accelerated mice). To do so, they injected the telomerase gene into the tails of the progeria mice. Not only did the telomerase prolong the lifespan of the aged mice by 20%, but it also reduced vascular impairments, like inflammation and DNA damage, demonstrating that telomerase therapy has anti-aging effects on mice.
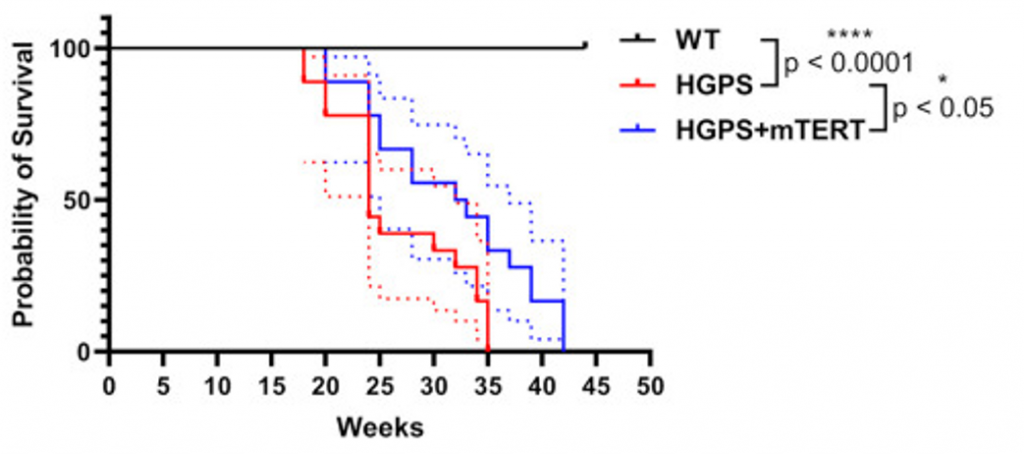
Previous studies have shown that telomerase gene therapy can extend lifespan and mitigate multiple conditions of aging in mice. Even a nasal gene therapy containing telomerase has been shown to increase mouse longevity. Mojiri and colleagues now show that the anti-aging effects of telomerase therapy may be due to reducing senescence. While senescence can possibly be mitigated by compounds called senolytics, which selectively eliminate senescent cells, another option for treating age-related conditions could be telomerase therapy.
Telomerase Anti-Aging Therapy
DNA damage activates our DNA repair systems, leading cells into a senescent state. When our telomeres shorten to a critical length, these DNA repair systems activate. Thus, it may be that shortened telomeres contribute to aging by inducing senescence. While telomerase is active during development, it is not present in most of our cells. Thus restoring telomerase to lengthen our telomeres provides an opportunity to prevent DNA damage, senescence, and aging.
A major problem with restoring telomerase is that it can potentially provoke tumor growth, as telomerase is activated in most cancer cells. In fact, there are currently clinical studies testing the effect of inhibiting telomerase to treat cancer. While telomerase gene therapy is one potential therapeutic option, telomerase activators are another. Telomerase activators can be found naturally in plants and include curcumin, resveratrol, and quercetin, all shown to have anti-aging effects. Such natural telomerase activators are also available as nutritional supplements.