Key Points
- In fish lacking the enzyme for telomere extension, telomerase, gut-specific activation of this gene can rescue premature aging.
- However, these fish have major reductions in lifespan compared to healthy, non-genetically altered fish.
- Activating telomerase in the gut of healthy fish doesn’t do much to improve their healthspan or lifespan.
In a new paper in Nature Aging called “Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish,” researchers say that “gut-specific rescue of telomerase activation leading to telomere elongation is sufficient to systemically counteract aging in zebrafish.” The key word in that sentence is “rescue,” which means that the experiments were done on fish whose telomerase gene was turned off.
While seven of the eight figures in this paper focused on the reintroduction of one copy of telomerase in fish that had both copies genetically removed and showed significant effects on healthspan and lifespan, only one figure showed the effects of increasing telomerase activity in healthy fish that already had two copies of the gene. This paper doesn’t show that turning on telomerase in a healthy environment can make people healthier or live longer.
Can Lengthening Telomeres Stop Aging?
Aging is characterized by telomeres getting shorter. Telomeres shield the ends of chromosomes from deterioration and detection by pathways in response to DNA damage. When telomeres are too short, DNA damage happens, which causes the cell cycle to stop, the cell to stop dividing, and the tissue to lose its integrity. A gene called telomerase can stop telomeres from getting shorter. Activation of the enzymatically active component of telomerase, called TERT, is restricted to stem cells.
Telomerase gene activation, on the other hand, is not enough to fully repair telomere erosion over the course of an organism’s lifetime. As a result, organisms that are getting older show signs of telomere dysfunction. People with telomerase or telomere maintenance protein gene mutations have telomeres that shorten too soon, a shorter life expectancy, and a group of diseases called telomere biology disorders. In the same way, telomerase depletion in zebrafish speeds up the shortening of telomeres. This causes telomerase-deficient animals to age faster and live fewer years. Zebrafish that don’t have telomerase show the same signs of dysfunction as zebrafish that age normally.
Both zebrafish that age normally and those that lack telomerase exhibit DNA damage from short telomeres in the gut, which is associated with reduced cell growth, an increase in senescent cells, and issues with how the body functions. Importantly, shortening of the telomeres causes cell and function problems in the gut at a time when other organs don’t have tissue problems. The zebrafish gut, like the human gut, has the fastest rate of telomere shortening. This causes early tissue dysfunction in both normal-aging zebrafish and telomerase mutants that age too quickly.
Severe problems with telomere biology are often linked to gastrointestinal syndromes. Patients with inflammatory bowel disease had shorter telomeres in their intestinal lining. So, the integrity of the telomeres is very important for gut health. But it is not known if the telomere-dependent aging of a single organ, like the gut, causes the body as a whole to age.
Telomerase Activation Has Minimal Anti-Aging Effects in Healthy Fish
Here, El Maï and colleagues show that minor but significant tissue-specific TERT activation in the gut can prevent telomere shortening and rescue premature aging in fish lacking TERT in all cells. Induction of TERT in these fish rescues gut senescence and low cell proliferation while restoring tissue integrity, inflammation, and age-dependent microbiota dysbiosis. Averting gut aging was shown to have systemic beneficial impacts, rescuing the aging of distant organs such as the reproductive and blood systems.
The research team, primarily consisting of researchers from the Université Côte d’Azur in France and the Instituto Gulbenkian de Ciência in Portugal, shows that gut-specific TERT activation extends the lifespan of fish lacking TERT by 40% while ameliorating natural aging. However, these fish had much shorter lifespans than fish with both copies of TERT intact.
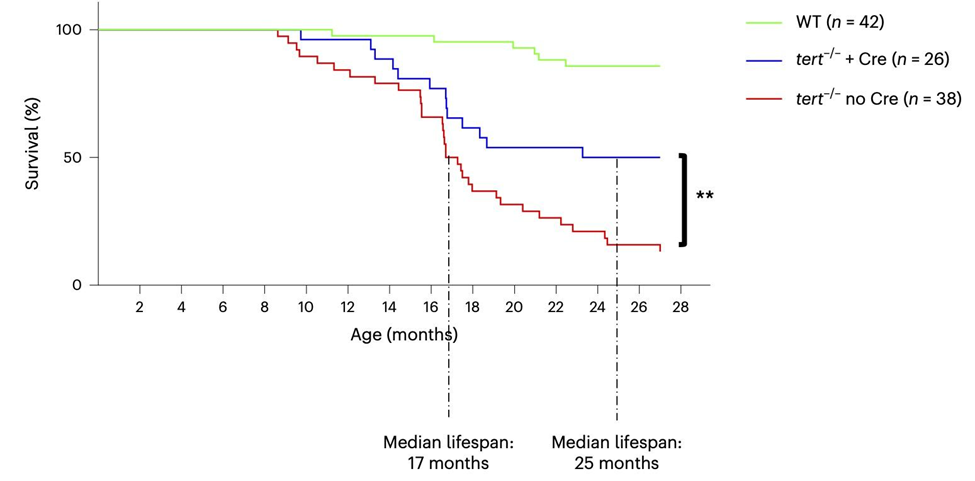
To extend their study to the natural aging of zebrafish, El Maï and colleagues studied zebrafish that still had both copies of TERT intact and also had activation of TERT in the gut. At 24-27 months, the researchers did not yet distinguish differences in survival between the two groups, which is when there were major differences in the fish lacking TERT.
The research team does claim to have observed that gut-specific TERT activation in fish increased cell proliferation and decreased cell replication arrest (senescence) in the gut compared to fish without activated TERT. They claim that, similar to their observations in fish lacking functional telomerase, activating TERT in the gut of WT fish is sufficient to improve the proliferative capacity of distant organs such as the testes and kidney marrow. These claims are based on the activation of proliferation genes in the testes and kidney marrow and not on the structure or function of these tissues. For example, there were no improvements in the number of sperm per testes.
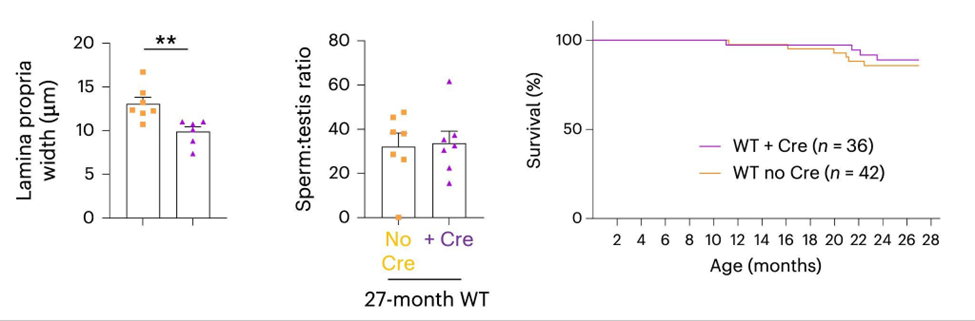
The researchers say that their data revealed that delaying gut aging by gut-specific TERT activation is sufficient to counteract the early signs of aging, such as loss of proliferative capacity, and that this also confirms that the gut is one of the earliest organs affected by natural aging. However, this claim as well as the statement “the most relevant systemic effect of gut-specific telomerase expression is lifespan extension while improving natural aging” are not very strongly supported by their data.
Telomerase Activation in Mice and Humans
While these results in fish are not exactly promising for those trying to follow potential breakthroughs in biotechnology to improve healthspan and lifespan, there is still hope because of consistent data in mice showing that activating telomerase in mice does significantly increase lifespan.
One 2019 study found that mice with hyperextended telomeres have less DNA damage with aging, are lean, show low cholesterol and LDL levels, have improved glucose and insulin tolerance, and also have a lower incidence of cancer and increased longevity. A 2021 study even went on to see what would happen if they delivered a virus that activates TERT intranasally or via injection. They found that the mouse cytomegalovirus (MCMV) carrying exogenous TERT extended the median lifespan by 41.4% and that this treatment significantly improved glucose tolerance, physical performance, as well as preventing body mass loss and alopecia. Further, telomere shortening associated with aging was ameliorated by TERT, and mitochondrial structure deterioration was halted in both treatments.
However, there isn’t much data yet in humans, and most of the data on TERT activation has been studied in human cancer cells. There are companies that are beginning to look at telomerase therapies, such as Telocyte, which is focusing on the development of telomerase therapy to treat Alzheimer’s disease. Additionally, there are registered trials looking at the use of chemical agents, such as cycloastragenol, on the reactivation of telomerase.