Key Points:
- Loss of the cell’s recycling process (autophagy) in human embryonic stem cells (hESCs) and neurons causes a metabolic defect and cell death.
- This cell death is due to the depletion of NAD+ through the hyperactivation of enzymes that consume NAD+.
- Agents that boost NAD+, such as NMN and NR, rescue the viability of autophagy-deficient human neurons.
Boosting NAD+ levels improves the health and survival of human brain cells that model neurodegeneration, according to researchers from the Whitehead Institute for Biomedical Research at MIT and the Institute of Cancer and Genomic Sciences at the University of Birmingham in the UK. The authors suggest that intervention with NAD+-boosting agents could have therapeutic relevance in a range of age-related, degenerative, or lysosomal storage diseases linked to autophagy dysfunction. This work was published in Cell Reports.
Alterations to Autophagy Affect the Brain
The process of autophagy essentially consists of the formation of a structure that picks up cargo and then fuses with another structure to degrade the cargo. This essential process clears undesirable molecules and cell structures that are then recycled for the use of energy and cell components. These functions of autophagy are vital for cell survival and pertinent for non-dividing cells like neurons, where the damaged cellular components are not diluted by cell reproduction.
When researchers create mutations to disrupt autophagy, specifically in the brains of mice or flies, cells of the central nervous system stop working or die—a process known as neurodegeneration. This also implies that autophagy is essential for maintaining the health of neurons. Despite the biomedical importance of autophagy in neurodegeneration, it is not clear how exactly autophagy causes this devastating process.
Rebalanced NAD+ Improves Survival of Autophagy-Deficient Neurons
Congxin Sun, Elena Seranova, and Malkiel A. Cohen led a research team that created a technique to look at dysfunctional autophagy in human cells to elucidate this causal link in a way that is pertinent to human health. The research team took human embryonic stem cells (hESCs), which can give rise to a range of human cell types like neurons, and created mutations similar to those used in mice to disrupt autophagy. In the neurons that grew and matured from the genetically altered hESCs, there were severe issues with metabolism and cell survival.
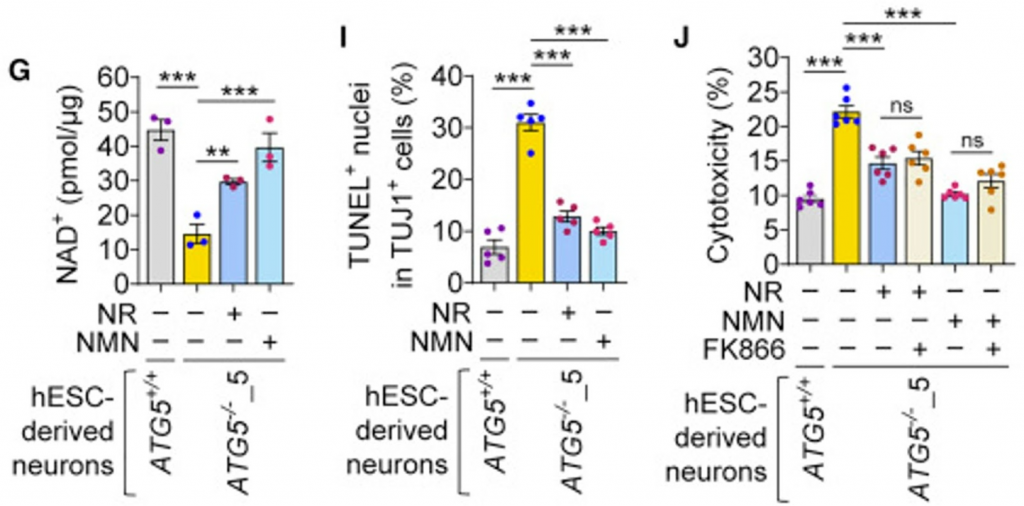
When they looked into what was happening at the molecular level in these cells, the researchers found that the levels of NAD+, a molecule essential for cells to make and use energy, were depleted. They found that this was due to excessive activation of enzymes that consume NAD+, called NADases.
When these cells were exposed to NAD+ precursors like nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), as well as other pharmacological agents, NAD+ levels went up and cell survival went up. Notably, NMN supplementation essentially recovered NAD+ levels to those of neurons from genetically unaltered hESCs and resulted in almost similar levels of cell death and toxicity. NR also recouped NAD+ levels and improved cell death and toxicity, albeit not to the same level as NMN.
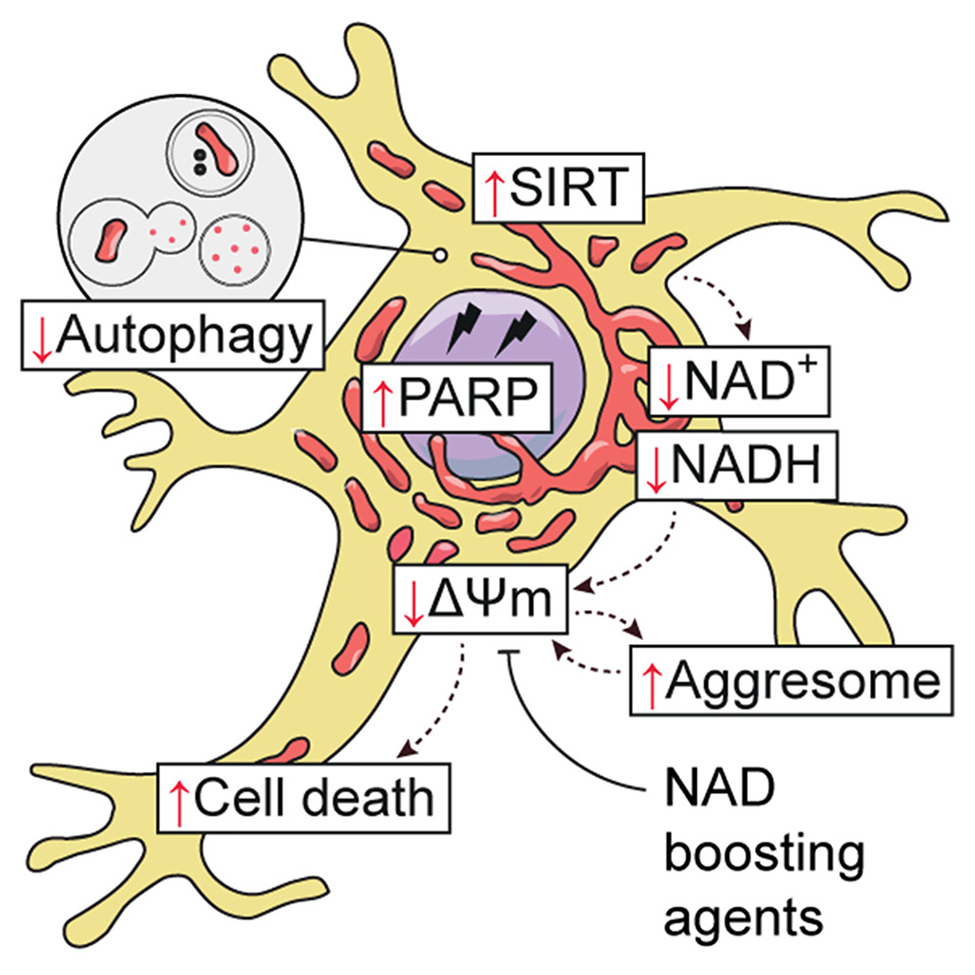
The hallmark of several neurodegenerative diseases in the aggregation of toxic proteins. Since autophagy is the primary clearance route for aggregated proteins, malfunction of this degradative process leads to stress from toxic protein buildup (proteotoxic) during aging and neurodegenerative diseases. Proteostasis then has a detrimental effect on the function of mitochondria, which are essential for many cell processes, including the production of energy.
In this study, neurons from genetically altered hESCs deficient in autophagy displayed a significant increase in protein aggregates and impacted mitochondrial health. But increasing NAD+ levels with either nicotinamide, NMN, or NR reduced the toxic buildup of proteins as well the negative effects on mitochondrial function.
Rebooting Autophagy in Aging and Disease
The authors suggest that this method of protecting cells by improving NAD+ levels may be directly applicable to people with Niemann-Pick type C1 disease, a neurodegenerative disorder linked to serious problems with autophagy. The authors conclude, “Our findings elucidate a mechanistic link between autophagy deficiency and neuronal cell death that can be targeted for therapeutic interventions in neurodegenerative and lysosomal storage diseases associated with autophagic defect.”
The relationship between NAD+ and autophagy extends beyond the nervous system and neurodegenerative diseases and into other tissue and organ systems in the context of aging. For example, kidney damage and diseases are common in the general population, especially in elderly persons. NAD+ is reduced in acute kidney injury (AKI) and chronic kidney disease (CKD), and mounting evidence indicates that NAD+ augmentation is beneficial to AKI, although conflicting results exist for cases of CKD. Reduced NAD+ levels in AKI compromise essential cellular pathways, including autophagy. For this reason, there have been calls for further studies on NAD+ in relation to AKI/CKD may shed light on novel therapeutics.
Researchers have also shown that therapeutic increases of NAD+ levels through the administration of NAD+ precursors or inhibitors of NAD+-consuming enzymes reactivate autophagy as well as improved metabolism, cell energetics, and inflammation in vascular cells—the cells lining blood vessels of humans and rodents with vascular diseases. Similarly, the regulation of NAD+ content is critical for maintaining autophagy in heart cells (cardiomyocytes), and the application of NAD+ precursors, such as NMN, has been shown to protect heart cells from damage. As such, NAD+ has emerged as a potential target for combatting age-related cardiovascular and cerebrovascular disorders.
One aging area that gets a little dicey is in the context of senescence, a phenomenon when cells stop growing and dividing often in aging. While senescence has been shown to work in a loop that drains NAD+—in which senescence-promoting molecules activate NADases that deplete NAD+ levels, triggering the presence of more senescence-promoting molecules—autophagy has been shown to both activate and inhibit senescence. So, there may be some cases where NAD+ boosting may need to be combined either with an autophagy inhibitor or activator, depending on how autophagy affects the aging of a particular cell type.