Key Points:
- NAD+ treatment following ischemic brain injury decreases damaged tissue area, increases neurological scores, and increases mouse survival.
- Treatment with NAD+ also decreases oxidative stress – cellular stress caused by increased reactive oxygen molecules – due to its effects on Sirt3.
- Inhibiting Sirt3 – a sirtuin protein found in the cell’s powerhouse and used to eliminate reactive oxygen molecules – prevents the NAD+-associated post-ischemia benefits.
The majority of strokes are caused when the blood vessels supplying the brain become blocked, leading to ischemia, or oxygen deprivation, in the brain tissue. The subsequent injuries following stroke – due to the brain being deprived of oxygen – can require vast amounts of extra care and therapies, decreasing patients’ quality of life. Stroke and subsequent injuries are quickly becoming a global problem as the population ages, with risks of stroke doubling nearly every 10 years after age 55. However, a recent study found that treating stroke patients immediately with NAD+ may be able to decrease ischemic injuries.
The study, out of China and published in Frontiers in Pharmacology, focused on mice undergoing strokes), subjecting their brains to oxygen deprivation. These mice were then treated with NAD+ – which has previously been shown to benefit neurodegenerative diseases – or butylphthalide (NBP) – a therapy currently undergoing research for treating stroke. Both treatments decreased the neurological deficits associated with ischemia, reduced the amount of dead tissue due to oxygen deprivation, and reduced the amount of fluid in the brain due to tissue injury. Additionally, oxidative stress was also decreased with treatment. NAD+ was shown to increase Sirt3 in the brain, suggesting that the effects on this mitochondrial protein may be the cause of many of the benefits that NAD+ has in ischemic brain injury.
NAD+ Decreases Injuries Due to Brain Oxygen Deprivation
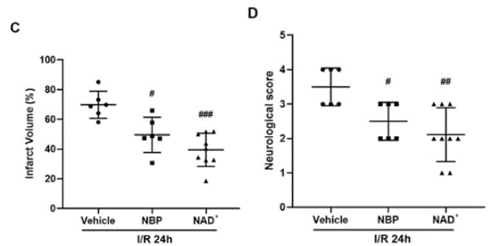
Previous research done by Wang and colleagues found that the ideal therapeutic doses of both NAD+ and NBP are 50 mg/kg and 30 mg/kg, respectively. They found that both treatments, at these doses, decreased the infarct volume – the area of dead tissue caused by oxygen deprivation due to the stroke – as compared to no treatment; however, NAD+ had a more profound effect. Additionally, both treatments improved neurological scores – based on a test developed to evaluate neurological functioning – and reduced fluid in the injured area.
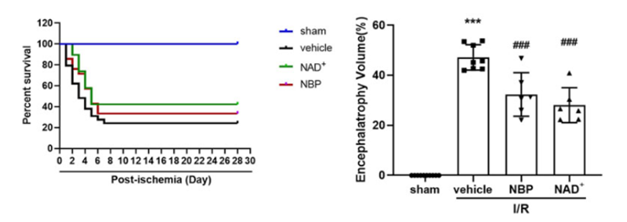
The investigators found that NAD+ and NBP both increased mouse survival by 42% and 33%, respectively, and helped to alleviate brain atrophy – the loss of nerve cells and brain tissue associated with brain injury or disease. Furthermore, both NAD+ and NBP helped to restore balance and coordination in mice following brain tissue oxygen deprivation and helped to improve learning and memory in the mice.
NAD+ Decreases Oxidative Stress Following Ischemia
NAD+ is known to improve the function of the mitochondria, which is essential for alleviating oxidative stress by increasing antioxidants and decreasing reactive oxygen species (ROS). Ischemia is a known cause of oxidative stress and ROS production, so the investigators looked at the effects of both NAD+ and NBP on oxidative stress. Both treatments reduced the oxidative stress seen following the ischemic event. Additionally, NAD+ treatment reduced DNA damage and other harmful events induced by oxidative stress, which could lead to cell death. NAD+ and NBP both prevented an increase in ROS following the ischemic event, with NAD+ yielding a slightly better antioxidant effect than NBP.
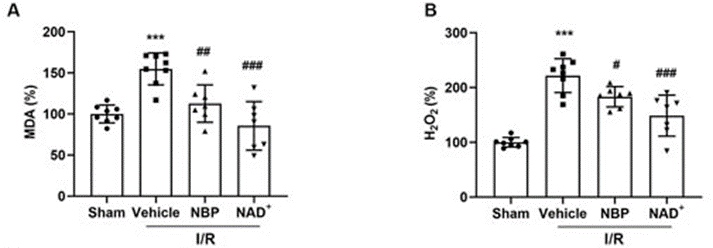
***p < 0.001 as compared to sham, #p < 0.05, ##p < 0.01 and ###p < 0.001 as compared to vehicle.
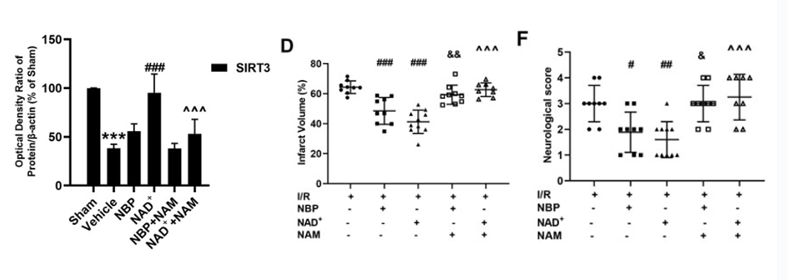
Sirt3 is part of the sirtuin family and resides in the mitochondria, where it helps to eliminate ROS and regulate other metabolic proteins. NAD+ is known to affect Sirt3 and other sirtuins. The investigators found that NAD+ treatment helped to increase Sirt3 in the ischemic tissue, while NBP had no effect on Sirt3. Furthermore, using a sirtuin inhibitor prior to NAD+ treatment negated all the NAD+-associated benefits, further suggesting that NAD+ alleviates brain injury and improves mitochondrial function and antioxidant effects following ischemia by increasing Sirt3.
NAD+, Sirtuins, and the Brain
Wang and colleagues show here how NAD+ can protect the brain from ischemia-associated injuries when given immediately following the event. It can improve neurological deficits and brain tissue due to its effects on Sirt3.
Sirtuins are becoming known for their longevity benefits (although there is still some controversy); however, as this study shows, their effects are far-reaching. Previous studies have shown that Sirt3 activation – through NAD+ supplementation – can help restore mitochondrial functioning following a heart attack. The same study showed that NAD+ can help rescue neurological deficits following a heart attack, similar to what was seen here with stroke.
However, the present study does have its limits given that NAD+ has to be given nearly immediately following the stroke, while in some cases, strokes go misdiagnosed. More studies need to be done to assess the effects of NAD+ on neurological deficits following a stroke, even after a long time has passed. However, given the effects that NAD+ has had on Parkinson’s patients and with ongoing trials in other neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and Friedreich’s Ataxia, there is still hope that NAD+ can be a neurological therapy breakthrough.