Key Points:
- Combining the NAD+ precursor NRH with chemotherapy enhances the effect of reducing brain tumor cell growth.
- NRH raises NAD+ levels in brain tumor cells, suggesting that boosting NAD+ helps prevent tumor growth.
The most common type of brain tumor is also the most lethal. Deadly tumors called glioblastomas spread rapidly in the brain and are nearly impossible to completely remove. For this reason, these aggressive brain tumors almost always grow back. A chemotherapy drug called temozolomide (TMZ) is used to prevent this regrowth, but some patients are resistant. Now scientists have found that boosting NAD+ may help restore TMZ’s tumor-killing capabilities.
Researchers from the MCI report in Cancers that resistance to brain tumor treatment can be largely prevented with the help of boosting NAD+ with one of its precursors, dihydronicotinamide riboside (NRH). They show that NRH helps diminish TMZ-resistant glioblastoma cell survival. They also show that NRH increases nicotinamide adenine dinucleotide (NAD+) levels in glioblastoma cells, suggesting that boosting NAD+ is responsible for enhancing tumor suppression.
NRH Aids in Preventing Brain Tumor Cell Growth
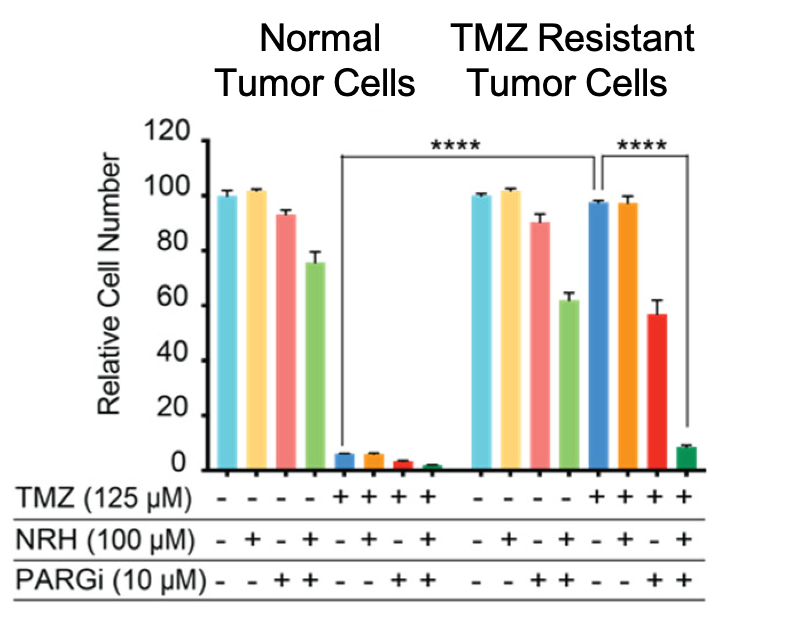
Scientists have been feverishly experimenting with new ways to overcome the low survival rate associated with glioblastomas and TMZ resistance. One solution has been to use drugs called PARG inhibitors (PARGi) in conjunction with TMZ. Li and colleagues previously discovered that NRH enhances PARGi, so they decided to combine PARGi and NRH (with TMZ) to determine if this combination prevents glioblastoma cell growth. They found that TMZ and PARGi inhibit tumor cell growth by less than 40% in TMZ-resistant cells. However, combining TMZ, PARGi, and NRH inhibits tumor cell growth by over 80%, revealing that NRH potentiates the effect of TMZ and PARGi to prevent tumor growth.
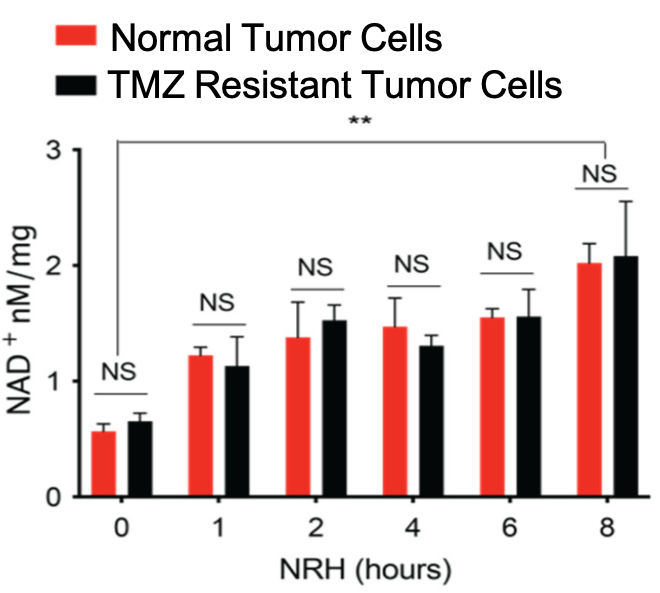
Since NRH is a precursor of NAD+, increasing NRH levels should increase NAD+ levels. To check for this, Liu and colleagues measured NAD+ levels in glioblastoma cells after NRH treatment. They found that NAD+ increased in response to NRH in both normal and TMZ-resistant glioblastoma cells, suggesting that the tumor-killing effect of NRH is from boosting NAD+.
Is NAD+ Good or Bad for Cancer?
Li and colleagues show that NRH dramatically increases PARGi’s toxicity against glioblastoma cells in the presence of TMZ. However, the Alabama scientists show that TMZ and NRH have no effect on reducing tumor growth unless combined with PARGi. Furthermore, a previous study showed that decreasing NAD+ increased TMZ-resistant glioblastoma growth, suggesting that without PARGi, boosting NAD+ could actually be harmful. This adds to the debate of whether increasing NAD+ levels promotes or hinders cancer growth.
The current research suggests that raising NAD+ levels in cancer cells themselves promotes cancer growth. However, boosting NAD+ can potentially treat other age-related diseases and possibly prevent cancer from developing. Additionally, as demonstrated by the findings of Li and colleagues, combining NAD+ boosters with other molecules like PARGi can enhance the toxicity of chemotherapeutics. This means that while increasing NAD+ in cancer cells may be bad, in combination with other therapies, NAD+ can be good.