Key Points:
- Treatment with NR prevents deficits in learning and memory in mice with brain injuries from reduced blood flow (ischemia).
- NR mitigates the brain damage caused by ischemic injury.
- NAD+ is increased in response to NR treatment, along with increased Sirt1 and AMPK activation.
When our arteries are blocked, the life-sustaining oxygen and nutrients within our blood cannot reach our cells, leading quickly to cellular energy reductions and cell death. This blocking of the arteries and lack of blood flow that often occurs with aging is called an ischemic stroke and if it occurs in the hippocampus — the primary region of the brain associated with learning and memory — it can lead to cognitive deficits. A new study shows that NAD+ — an essential molecule associated with maintaining energy homeostasis — may help alleviate the damage caused by ischemic injury.
In Neurochemical Research, Cheng and colleagues from Huazhong University of Science and Technology in China show that treatment with NR reduces hippocampal damage and preserves cognitive function in a mouse model for ischemic stroke. Specifically, they show that NR treatment improves learning and memory, infarct — dead tissue — size, neuronal abnormalities, and cell death. They also show that NR raises NAD+ and ATP — cellular energy molecules — deficits associated with ischemic injury. Furthermore, they show an increase in the activity of energy modulators Sirt1 and AMPK. These findings suggest that boosting NAD+ with NR could potentially reduce the brain damage and cognitive deficits associated with ischemic stroke.
NR Improves Ischemia-Induced Cognitive Deficits & Brain Damage
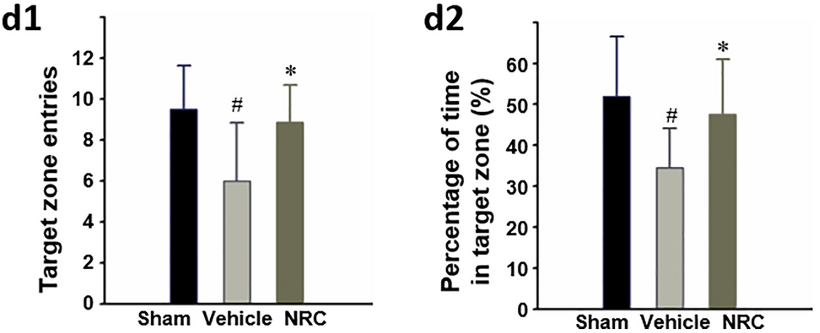
To determine the effects of NR — a natural NAD+ precursor that raises NAD+ levels — on ischemic injury, Cheng and colleagues induced ischemia (lack of blood supply) in the hippocampus of mice. To test learning and memory, they used the Morris water maze test. In doing so, mice were trained over several trials to find a platform in a pool of water to escape from drowning. In the final trial, the platform was removed and the proportion of time spent by the mice in the previous location of the platform was measured to assess learning and memory. Cheng and colleagues found that ischemic mice treated with NR spent more time in the correct location compared to untreated ischemic mice, indicating improvements in learning and memory.
Upon ischemia, a lack of blood leads to a cellular energy crisis, whereby mitochondria do not have the oxygen necessary to produce ATP, collapsing the intricate order of the cell and leading to cell death. This begins locally with only a few cells at the initial site of the occlusion but spreads as the survival of each cell depends on the next. This spread of dead cells determines the size of an infarct, which increases with time, making time a critical factor.
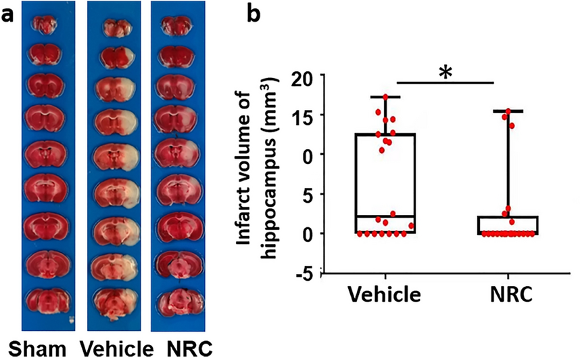
Cheng and colleagues examined mouse hippocampus infarct size (volume) three days after ischemic injury and found that NR treatment reduces the spread of dead neurons, as shown by a reduction in the infarct size. Additional measurements showed that NR treatment partially reverses neuronal damage and suppresses cell death. These findings suggest that NR helps to maintain cellular energy and slow down the spread of cell death upon ischemic injury.
NR Helps Prevent a Cellular Energy Crisis
NR is known to activate enzymes called Sirt1 and AMPK, which restore energy balance in times of energy crisis by stimulating ATP production. NR likely does this by increasing the synthesis of NAD+, which Sirt1 uses as fuel. Sirt1 then activates AMPK. NAD+ itself also plays an essential role in mitochondrial ATP production. Thus, by boosting NAD+ and activating Sirt1 and AMPK, NR could exert its effects on ischemic injury by preventing a cellular energy crisis.
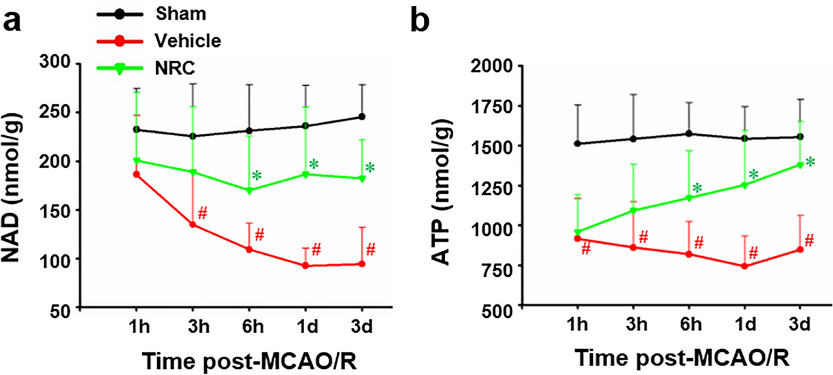
Cheng and colleagues measured NAD+, ATP, Sirt1, and AMPK at different time points from the hippocampus of mice after ischemic injury. Upon ischemic injury, NAD+ and ATP levels dropped with a 40% decrease in ATP, confirming a cellular energy crisis. NR treatment replenished the production of NAD+ and ATP six hours after injury. Sirt1 and AMPK activity also increased after 6 hours. These results suggest that by increasing the production of NAD+, treatment with NR helped salvage the energy supply by activating Sirt1/AMPK.
Timing Is Everything
When it comes to stroke, they say “time is brain.” When an individual has a stroke, the blocked artery must be repaired with reperfusion therapy, which restores blood flow to the site of occlusion. Until the artery is unblocked, more and more brain tissue is damaged. Thus, time is a critical factor in preventing brain damage for stroke victims. For the model used by Cheng and colleagues, reperfusion occurred 45 minutes after ischemic injury. NR treatment was then given 20 minutes after reperfusion. In humans, reperfusion may not occur for hours. Still, NR could help reduce brain damage after reperfusion or it could be administered before reperfusion. Since NR is not a fast-activating compound, taking 6 hours to have a significant effect in this study, future studies will need to examine the most critical time points for NR administration in the case of ischemic injury.
Is NR a Neuroprotective Treatment?
This study confirms what was found in a similar study using another NAD+ booster, NMN. In previous animal studies, NR has also been shown to improve cognitive function and increase lifespan. NR has been shown to rescue neurons in cell models for Alzheimer’s disease and improve neuron generation in an amyotrophic lateral sclerosis mouse model. These studies support the neuroprotective effect of NR observed in this study. NR treatment has been shown to raise NAD+ levels in humans, which could make it applicable in treating cognitive deficits in humans. Additionally, NR has been shown to improve the underlying defects of Parkinson’s disease. Overall, NR could have more of a preventative effect on the cognitive decline associated with aging, as NAD+ levels decline with age.