Key Points:
- Compounds that improve mitochondrial dynamics – the fission (splitting) and fusion of mitochondria – increase cell survival in mice cells modeling Alzheimer’s disease.
- These compounds also increased the levels of proteins critical for signal transmission between neurons.
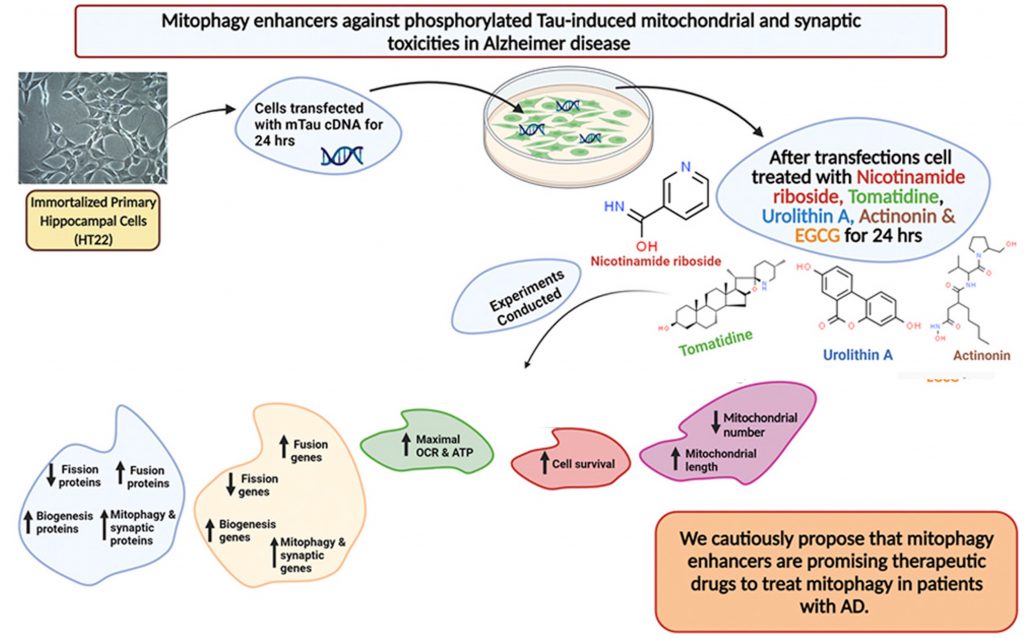
Alzheimer’s disease (AD) has no cure, yet is one of the most prevalent neurodegenerative disorders among the global aging population, ranking 6th in the world for the leading cause of death. In attempts to slow the progression of AD, scientists have honed in on the importance of keeping a healthy balance of our cell’s power generators, the mitochondria. The clearance of defective/dead mitochondria, a process known as mitophagy, is essential for metabolism and has been shown to alleviate cognitive deficits. Thus, researchers are exploring how mitophagy-inducing compounds (mitophagy enhancers) affect overall brain function.
Texas Tech University researchers published an article in Pharmacological Research showing that mitophagy enhancers improve nerve connections (synapses) and cell survival in mouse cells that model AD. Reddy and colleagues showed that treating these cells with compounds – including NAD+ boosters and nicotinamide riboside and urolithin A – that improve mitochondrial dynamics and function increased proteins critical for synaptic activity compared to untreated AD mouse cells. These findings give insight into the possibility of reducing mitochondrial and synaptic abnormalities in patients with AD utilizing specific mitophagy enhancers.
(Reddy et al., 2021 | Pharmacological Research)
The Link Between Mitochondrial Abnormalities and Alzheimer’s
One of the hallmarks of AD progression is the accumulation of phosphorylated tau (P-tau), which forms structures called “tangles” in neurons that disrupt neuronal communication. Current data suggest that P-tau accumulation hampers mitochondrial distribution between neurons by impairing the transport of organelles like mitochondria between the neuron’s cell body down to the synapse, a process critical for neurons to communicate.
P-tau has also been linked to subpar mitochondrial dynamics, facilitating neuronal damage by compromising mitochondrial structure and function. Research has made it clear that healthy mitochondria are the metabolic pillars that require continual support to prevent the development of various age-related diseases, particularly AD. As a result, scientists continue to pursue research that is aimed towards limiting mitochondrial toxicities.
Mitophagy Enhancers Improve Mitochondrial Dynamics in AD
To identify a therapeutic strategy that may mitigate damage caused by P-tau in AD, Reddy and colleagues examined the potential of different compounds linked to improvements in mitophagy – the clearance of damaged and dysfunctional mitochondria. To maintain a healthy pool of mitochondria, there must be relatively equal fission and fusion reactions. When fission reactions surpass the normal threshold, the pool of mitochondria becomes compromised, which has been shown to drive the development of various neurodegenerative diseases.
With this in mind, Reddy and colleagues looked at several compounds linked to mitophagy. Two of these compounds – nicotinamide riboside and urolithin A – are thought to influence mitophagy by boosting NAD+ levels. The other two compounds that the Texas Tech researchers examined were the anti-microbial actinonin and Tomatidine. Using an AD modeling mouse hippocampal cells (mTau-HT22), they looked at the levels of proteins involved in the fission and fusion of mitochondria before and after treatment with these mitophagy-enhancing compounds.
Prior to treatment, mTau-HT22 cells exhibited lower levels of fusion proteins and elevated levels of fission proteins, characteristic of impaired mitochondrial dynamics. Upon treating mTau-HT22 cells with mitophagy enhancers, fusion protein levels showed significant increases while fission proteins returned to normal levels, indicating improved mitochondrial dynamics. Of note, investigators found that urolithin A was the mitophagy enhancer that performed best at restoring mitochondrial dynamics.
Notably, sufficient ATP levels are critical to sustain brain cellular functions and limit overall toxicities. Thus, Reddy and colleagues examined the effects of mitophagy enhancers on the primary function of mitochondria – mitochondrial respiration, the cellular process responsible for converting macronutrients into the energy (ATP) that powers all cells. As expected, mitophagy enhancers promoted the highest oxygen consumption rate (OCR) in mTau-HT22 cells, which was indicative of elevated mitochondrial respiration. Taken together, these results further support the potential use of mitophagy enhancers towards mitochondrial and synaptic toxicities found in patients with AD.
Mitophagy Enhancers Boost Synaptic Activity and Cell Survival
Given that synaptic activity drastically declines with AD, the Texas researchers tested for levels of a synaptic protein called synaptophysin in mTau-HT22 cells following treatment. Initial observations showed that mTau-HT22 cells had significantly lower levels of synaptophysin than control cells. But after treating mTau-HT22 cells with mitophagy enhancers, synaptophysin levels surpassed the concentrations seen in both control and untreated cells, suggesting increased synaptic activity.
Moving forward, the Texas researchers examined mTau-HT22 cell survival following treatment to further analyze the protective effects of mitophagy enhancers. Results showed that all mitophagy compounds increased mTau-HT22 cell survival, with urolithin A, again, outperforming the other mitophagy enhancers. These initial findings show that mitophagy enhancers successfully mitigate mitochondrial toxicities by maintaining balanced mitochondrial dynamics and increasing cell survival.
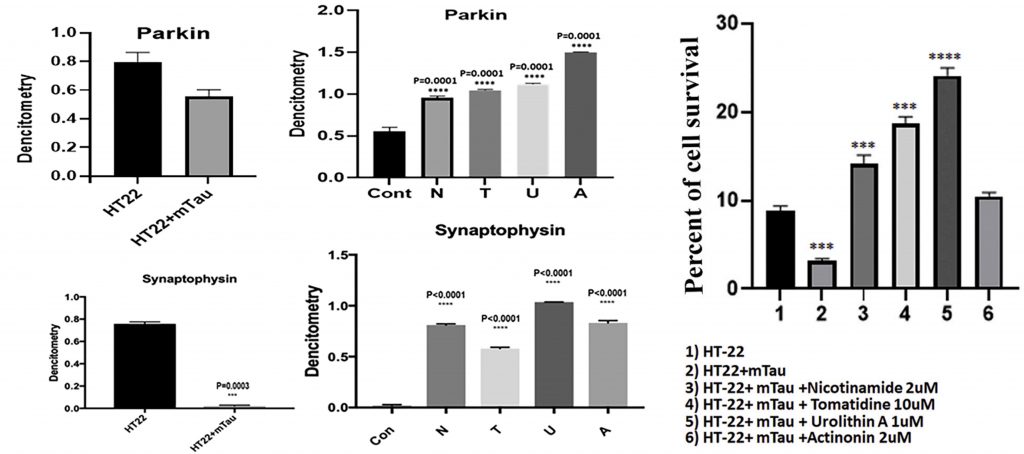
(Reddy et al., 2021 | Pharmacological Research) Mitophagy enhancers increase mTau-HT22 cell survival and synaptic proteins. The left four panels show how the levels of two proteins integral to synaptic connection and function, parkin (top) and synaptophysin (bottom), decrease in hippocampal mouse cells modeling AD (HT22+mTau) compared to unaffected hippocampal cells (HT22). Treatment with mitophagy enhancers increases the levels of these synaptic proteins. N, nicotinamide riboside; T, tomatidine; U, urolithin A; A, actinonin.The graph on the right shows the percentage of mTau-HT22 cell survival in control cells (1), untreated mTau-HT22 cells (2), and mTau-HT22 cells treated with mitophagy enhancers (3-6). Urolithin A promotes the most cell survival.
The Potential Of Mitophagy Enhancers
Researchers continue to unravel the full potential of mitophagy-inducing compounds. Even the mother of Elon Musk, Maye Musk, is using a mitophagy enhancer as the main ingredient in her new anti-aging supplement Mitopure. While scientists continue to highlight the critical advantages of utilizing mitophagy-inducing compounds to combat the progression of age-related diseases, recent studies have also demonstrated their effectiveness towards microbiome health and muscle maintenance in older adults.
Let’s not forget that these compounds are potent NAD+ boosters, known to promote DNA repair, improve cardiac functions, and protect against age-associated metabolic disorders. All things considered, it’s evident that mitophagy enhancers have a definitive role in helping maintain overall health and metabolism.