Key Points:
- CAR T cell treatment reduces fibrosis in mice modeling heart failure.
- Injured mice treated with CAR T cells exhibit normal heart function.
- One week following CAR T cell treatment, all CAR T cells dissipate completely.
When our hearts succumb to injury, our cells kick into high gear and activate cells that make up our connective tissue (fibroblasts), generating fibrous scar tissue in a process known as fibrosis. However, the continual accumulation of fibrous scar tissue rigifies the heart muscle, diminishing overall performance and contributing to the development of various deadly heart conditions. And with heart disease being the number one killer in the US, scientists must continue to seek new treatment modalities.
A new study published in Science by University of Pennsylvania researchers details the novel use of mRNA technology to treat fibrotic scars in mice modeling heart failure. Here, Epstein and colleagues delivered modified mRNA into mice to engineer immune fighting cells called CAR T cells instructed to block fibroblast activation, a known promoter of fibrosis. Through transient generation of CAR T cells, mice displayed improvements in fibrosis and heart function. Together, the study’s findings confirm the potential of implementing mRNA technology as a targeted therapeutic for all kinds of diseases, not just those involving fibrosis.
“Fibrosis underlies many serious disorders, including heart failure, liver disease, and kidney failure, and this technology could turn out to be a scalable and affordable way to address an enormous medical burden. But the most notable advancement is the ability to engineer T cells for a specific clinical application without having to take them out of the patient’s body,” said senior author Jonathan A. Epstein in a press release.
What Are CAR T Cells? How Are They Used?
Until recently, scientists had to manually remove T cells from a patient’s blood before undergoing genetic modification in the lab to generate CAR T cells before being reinfused into the patient. Not only does this approach require exorbitant amounts of money and resources, but the produced CAR T cells can persist for months or even years in the body, which can be counterproductive when treating fibrotic diseases. If CAR T cells continually block fibroblast activation, which is required for wound healing, then sustaining future injuries following treatment poses an extreme health risk. Fortunately, the newly developed CAR T cell therapy, which occurs within the body of the living animal (in vivo), circumvents this issue.
The multifaceted potential of in vivo CAR T cell therapy strikes excitement to the scientific community. By using small spherical vesicles called lipid nanoparticles to deliver genetically altered mRNA into T cells, scientists bypass previous CAR T cell procedures and directly reprogram CAR T cells to target any disease-linked protein they want. Moreover, these CAR T cells completely dissipate one week after treatment, which allows for fibroblast activation and, thus, treatment of future injuries.
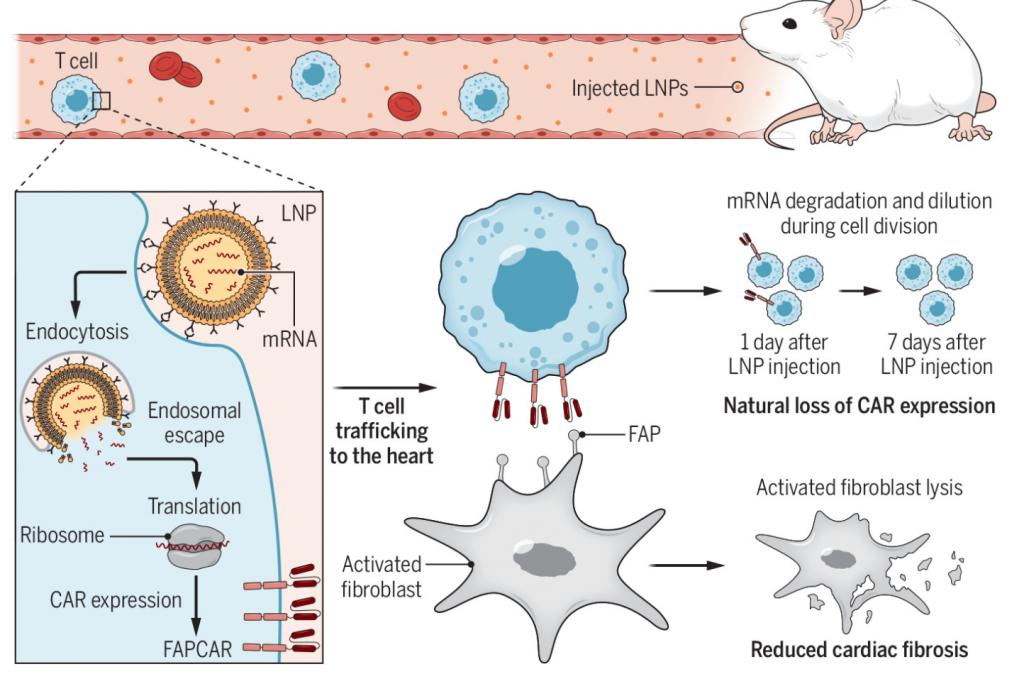
(Chen et al., 2022 | Science) Generation of transient engineered T cells in mice. To produce CAR T cells in vivo, lipid nanoparticles (LNPs) filled with genetically modified mRNA are injected into mice and delivered to T cells. T cells absorb the LNPs via endocytosis. mRNA is then released and undergoes translation to synthesize the fibroblast activation protein chimeric antigen receptor (FAPCAR). The newly generated CAR T cells then migrate to the heart and deactivate FAP, leading to reduced cardiac fibrosis. One week after LNP injection, the CARs completely degrade, leaving normal T cells.
CAR T Cell Treatment Boosts Heart Function and Minimizes Fibrosis
To induce fibrosis in the heart, Epstein and colleagues orally supplemented mice with the following two compounds: angiotensin II and phenylephrine. One week after fibrotic development, injured mice were either injected with the lipid nanoparticles responsible for CAR T cell synthesis or a controlled saline solution. Investigators then waited another two weeks before fibrosis evaluation. Upon initial observations, injured mice injected with saline solution displayed the most fibrosis. And although uninjured control mice showed the least fibrosis, injured mice injected with lipid nanoparticles presented significantly less fibrotic tissue than injured mice injected with saline solution, which suggests CAR T cells are deactivating fibroblasts and limiting cardiac fibrosis.
Next, the research team looked at heart parameters indicative of normal heart function: end-diastolic volume, end-systolic volume, E/e’, and ejection fraction. The findings showed that injured mice injected with saline solution exhibited abnormal values in each measured parameter, indicating that fibrosis negatively affects overall heart performance. Meanwhile, injured treated mice produced values nearly equal to uninjured control mice, further highlighting that CAR T cells restore heart function while simultaneously eliminating cardiac fibrosis.
Lastly, Epstein and colleagues confirmed the transient nature of the tested CAR T cells. One week after treatment, investigators failed to detect any significant levels of CAR T cells, meaning that injured mice still possess the necessary tools to heal future injuries.
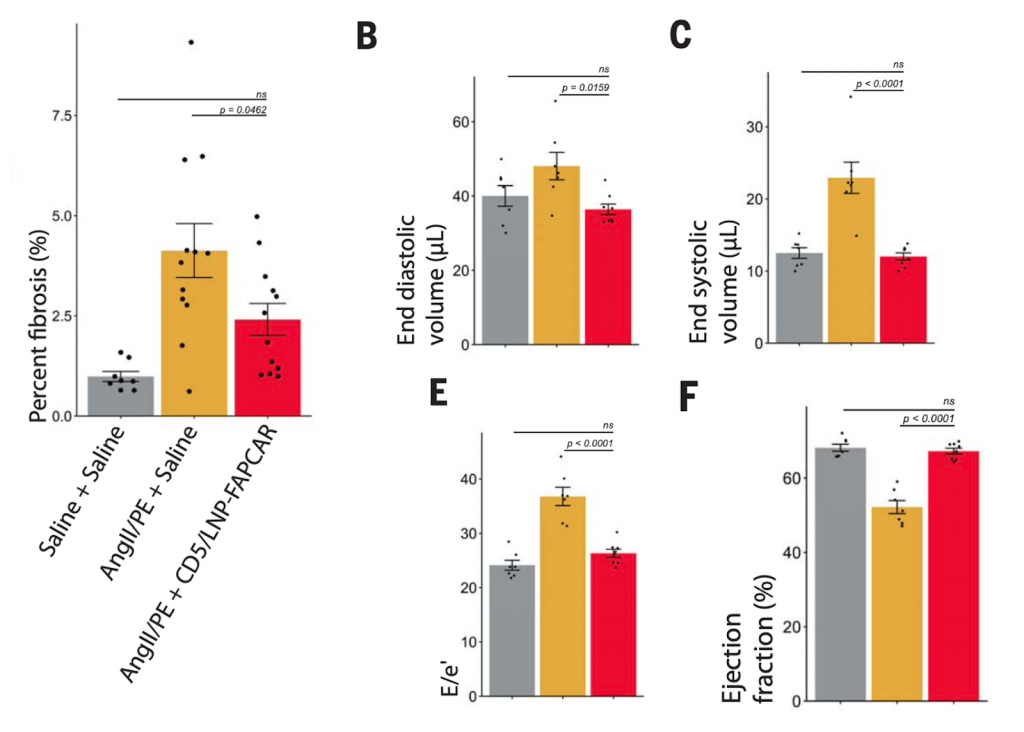
(Epstein et al., 2022 | Science) CAR T cell therapy lowers cardiac fibrosis and enhances heart function. Experimental mice were divided into three groups: uninjured control (Saline + Saline), injured-no treatment (AngII/PE + Saline), injured-treatment (AngII/PE + CD5/LNP-FAPCAR). The graph on the left shows the amount of fibrosis in each experimental group. Graphs b,c,e, and f display various heart parameters to characterize the overall performance in each experimental group.
The Potential of In Vivo CAR T Cell Therapy
Notably, the technology used in this study is responsible for creating the SARS-CoV-2 vaccine, which, when taken, provides our cells with instructions to produce the necessary antibodies to fight off the virus. So, the fact that scientists used this mRNA technology to create the covid vaccine in record time suggests that we may be able to use the same procedures to develop other disease therapeutics sooner than we hoped for.
“Nevertheless, the possibility of an ‘off-the-shelf’ universal therapeutic capable of engineering specific immune functions provides promise for a scalable and affordable avenue to address the enormous medical burden of heart failure and other fibrotic disorders,” concluded Epstein and colleagues.