Key Points:
- Immune cells called T cells can retain the potential for extraordinary population expansion and longevity well beyond their organismal lifespan.
- These immune cells can replicate 1040 times without becoming functionally exhausted
- The findings have major clinical implications for the growing field of cell-based therapies and aging.
Can cells avoid aging, or is aging an unavoidable, programmed fate?
A new study published in Nature shows that descendents from immune cells can be continuously harvested and transferred into new mice for over 10 years, which is about four times the lifespan of a mouse. Despite demonstrating the potential to expand the starting population to at least 10 duodecillion-fold (or a billion times a billion times a billion times a billion), the immune cells did not show loss of replication control. “We calculated that the T cells in this study had the potential to produce enough progeny to occupy a volume 30,000 times greater than that of Earth,” wrote authors David Masopust and Andrew G. Soerens.
These results show that immune cell senescence, which is when cell replication stops because of aging, can be avoided and that cells can live much longer than their host. Aging and longevity savant David Sinclair, professor of genetics at Harvard Medical School and the co-director of its Paul F. Glenn Center for Biology of Aging Research, said that this study shows that “immune cells can retain the potential for extraordinary longevity well beyond their organismal lifespan.”
Is the “Hayflick Limit” a Hoax?
The extent to which non-reproductive (somatic) mammalian cells can replicate has been long debated. Hayflick and Moorehead published a paper in 1961 that changed the way people thought about somatic cell immortality. In it, they said that a division counter showed that human cells could only divide 50–60 times. This is called the ‘Hayflick limit’ and was proposed to work to impede the development of cancer but at the cost of imposing lifespan constraints.
Curiously, CD8+ T lymphocytes, immune cells critical for battling an infection, can remain dormant for years yet activates within minutes and quickly replicate—a mechanism at the core of our immune response. Data suggests that T cells in mice responding to chronic infections or cancer undergo progressive ‘exhaustion’, defined by impaired replicative ability and failure to eliminate immune targets. But whether T cell division potential is intrinsically finite or unlimited has been unclear.
Since the 1970s, there have been reports that T cells could occasionally be sustained in culture for long periods of time. But the data so far point to a model in which T cells have a natural limit to how many copies they can make.
From One Cell to A World’s Worth
In this study, senior author Andrew G. Soerens and his team injected mice with three different viruses. Each virus made a molecule that the same group of T cells could recognize. This triggered the T cells, which led to a large and long-lasting increase in the number of cells that could respond to the viral molecule. The cells from the expanded population were transplanted into fresh mice and multiplied once more using the same virus immunizations. T cells were transferred once more using mice from the initial batch, and this procedure was repeated repeatedly. T cells proliferated vigorously in response to vaccination over the course of 10 years (roughly 4 mouse lifespans), 16 cell transfers, and 51 total immunizations.
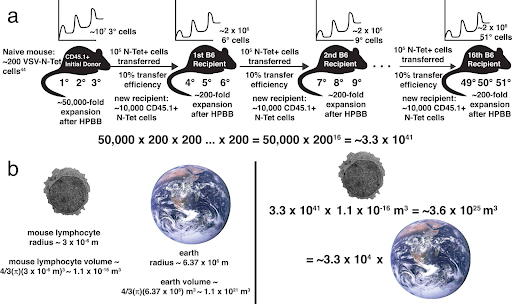
This method showed that T cells could increase their number by at least 1040 times from where they started. The authors said that the results were not messed up by young cells, which means that the T-cell population that was specific to the first immunization was much older than a typical mouse’s lifespan. However, the T cells required a period of rest between immune-stimulation events. Even though the T cells that were repeatedly boosted kept working as immune cells, they also slowly started to show molecular signs of T-cell exhaustion. Notably, these T cells did not grow out of control. They still needed the antigen to make them grow quickly before returning to a state of rest.
A Sell for Cell Therapies
The results show that T-cell senescence is not a natural result of repeated cell growth. Also, these results have important clinical implications for the growing field of cell-based therapies. In these therapies, immune cells are taken from the body and changed to treat diseases like cancer and infections. These findings suggest that, if prepared just right, patients would need just a single treatment with cell therapies using immune cells to fight off disease.