Key Points:
- Markers of DNA damage, inflammation, and senescence, all of which are hallmarks of aging, are suppressed in the brains of cocktail-treated mice.
- The findings reveal that targeting multiple biological pathways – a series of interactions between molecules to serve a specific function – may be more beneficial than targeting one pathway alone.
Aging is multifaceted and complex, involving a myriad of interacting biological pathways that gradually collapse and lead to organ and tissue dysfunction. Many of the current anti-aging therapeutics target only one specific biological pathway, narrowing their impact on slowing, preventing, or reversing aging. This is why scientists have turned to drug combinations that can potentially widen the scope of treatment by targeting multiple biological pathways.
Previously, University of Washington researchers showed that a drug cocktail of rapamycin, acarbose, and phenylbutyrate attenuated features of aging, including cognitive decline in mice. Now, in a preprinted (in the process of being peer-reviewed) article published in bioRxiv, Jiang and colleagues explore the mechanisms behind the cocktail’s protective effects against cognitive decline. After harvesting and examining the brain tissue of mice, the investigators found that the cognitive-boosting cocktail regulates pathways linked to aging, particularly those involved in DNA damage, inflammation, and senescence. What’s more, analysis of gene activity confirmed that the cocktail targets the genes that regulate these pathways.
Cognitive-Boosting Cocktail Regulates Aging Pathways
The continual stress our cells accumulate with age eventually triggers a state known as cellular senescence – a permanent growth-arrested state that drives the development of many age-related diseases like heart disease, sarcopenia (muscle decline), and neurodegenerative disorders like Alzheimer’s, and Parkinson’s disease. Furthermore, the buildup of senescent cells upon aging ignites inflammation, another hallmark of aging that underlies age-related cognitive decline. With this in mind, Jiang and colleagues explored whether the cognitive-boosting combination of rapamycin (Rap), acarbose (Acb), and phenylbutyrate (PBA) target metabolic pathways tied to senescence and inflammation.
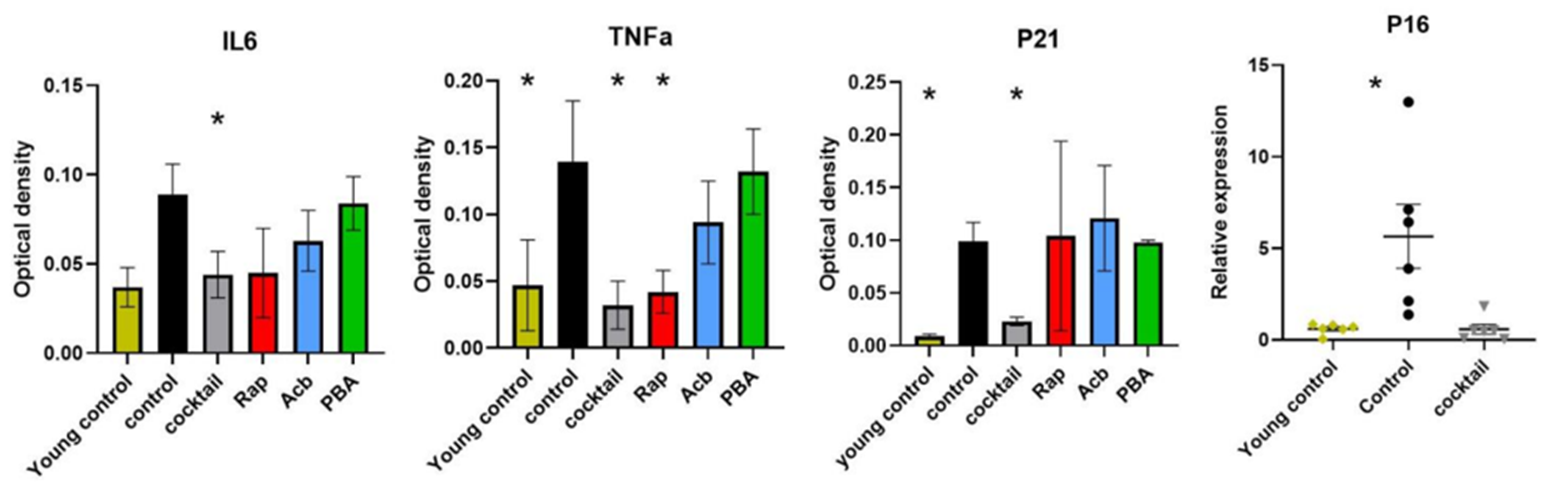
To test this, the investigators harvested the brains of mice subjected to cognitive tests from the previous study and examined the activity of their mRNA – molecules that provide instructions for protein synthesis. Jiang and colleagues showed that cocktail-treated mice displayed significantly lower P16 and P21 levels, both of which are well-established markers of senescence. Notably, senescent cells secrete molecules collectively known as the senescence secretory phenotype (SASP) that drive inflammation and stimulate the formation of new senescent cells. The results showed that cocktail-treated mice exhibit reduced activity of SASP pathways.
The investigators also found that treating middle-aged mice with the cocktail decreased the activity of pro-inflammatory pathways and significantly reduced the levels of IL-6 and TNFɑ, two proteins linked to chronic inflammation. Taken together, the findings suggest that the synergetic cocktail boosts cognition by regulating senescent and inflammatory pathways known to drive aging.
A vicious cycle begins when aging triggers senescence. As we age, our genetic blueprints (DNA) become compromised, and research suggests that DNA damage is central to increased senescent cell burden. The accumulation of senescent cells inevitably increases inflammation and oxidative stress, which further drives DNA damage and restarts the cycle.
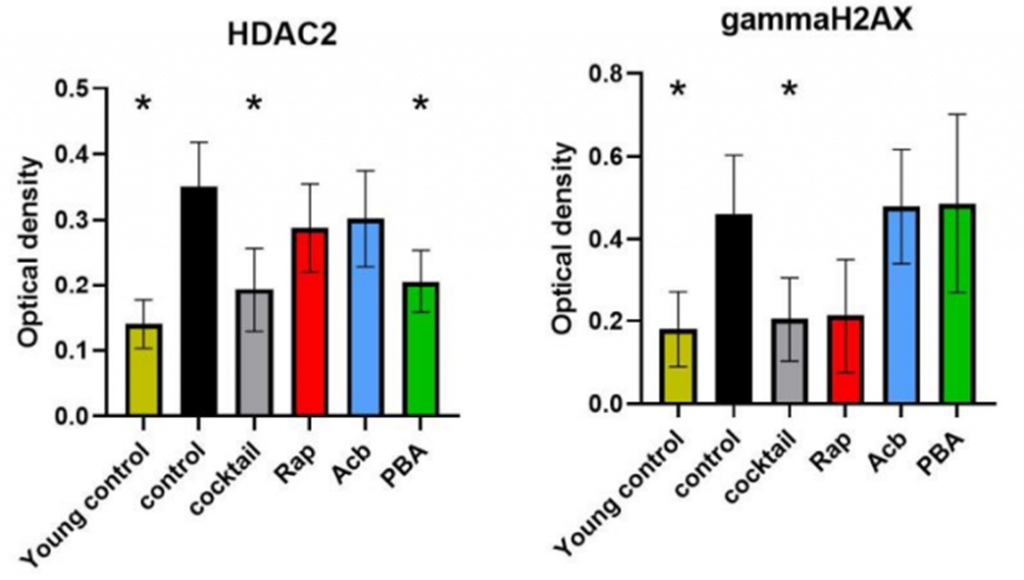
Jiang and colleagues proceeded to analyze proteins indicative of DNA damage and found that cocktail-treated mice exhibit drastically lower levels of two key proteins (HDAC2 and gammaH2AX), indicating that the cocktail mitigates senescence and inflammation by protecting DNA. Overall, the study elucidates the potential mechanisms behind the cognitive-boosting effects of the synergetic cocktail and suggests that combining treatments may be more effective than individual treatments at delaying features of aging.
Combining Treatments for Synergy
In this study, all drugs making up the cognitive-boosting cocktail are FDA approved for people with clinical conditions and have known benefits of increased healthspan and prolonged longevity. Moreover, the study’s findings shed light on the potential of future synergizing therapies against cognitive decline and other age-related diseases. Given that many biological pathways are intertwined, drug combinations such as the one in this study have the potential to target multiple pathways simultaneously, which would likely contribute to a more robust alleviation of complications from age-related diseases. That being said, it may be worth exploring whether certain drug combinations elicit negative responses to paint a clearer picture of which drugs work in synergy and which do not.