Highlights:
- In a phase 3 clinical trial evaluating the efficacy and safety of lecanemab, researchers successfully show that the drug delays Alzheimer’s related cognitive impairment and reduces the accumulation of amyloid beta plaques – proteins believed to be a primary driver of Alzheimer’s.
- The trial’s findings convinced the FDA to grant lecanemab traditional approval for the treatment of Alzheimer’s.
In the realm of neurodegenerative disorders, Alzheimer’s is arguably one of the most devastating diseases, afflicting more than 6.5 million people in the U.S. alone. And although no real cure exists, the available research suggests that compounds capable of extinguishing amyloid beta plaques are promising therapeutics to mitigate this deadly disease. Accordingly, preliminary clinical trials have established the drug lecanemab as a potent inhibitor of amyloid plaque buildup, demonstrating its potential to treat Alzheimer’s.
In January of 2023, lecanemab was granted “accelerated approval” by the U.S. Food and Drug Administration (FDA) to treat Alzheimer’s patients. This particular status/approval is usually given to drugs when there is an unmet medical need, and the drug’s benefits are expected to outweigh its risks. However, in June of 2023, the positive results of Study 301 (CLARITY AD), a Phase 3 randomized, controlled clinical trial that evaluated the safety and efficacy of lecanemab in patients with mild cognitive impairment and Alzheirmer’s-induced dementia, prompted the FDA to shift lecanemab’s status from “accelerated approval” to “traditional approval,” marking a significant milestone in its utilization for Alzheimer’s disease therapy.
“Today’s action is the first verification that a drug targeting the underlying disease process of Alzheimer’s disease has shown clinical benefit in this devastating disease,” said Teresa Buracchio, acting director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research. “This confirmatory study verified that it is a safe and effective treatment for patients with Alzheimer’s disease.”
Lecanemab Delays Cognitive Decline and Blunts Amyloid Plaque Buildup
The nature of Alzheimer’s is multifaceted and complex; however, countless studies have pointed to amyloid plaque aggregation as a key trigger of Alzheimer’s. Moreover, the gradual accumulation of amyloid plaques upon aging is thought to ignite inflammation, destroy neurons, and compromise synapses – neuronal junctions that allow electrical or chemical signals to be transmitted between neurons. Lecanemab is one of the few drugs capable of ameliorating these contributing factors of impaired memory and cognition, and Study 301 further elucidates the clinical benefits of lecanemab in Alzheimer’s patients.
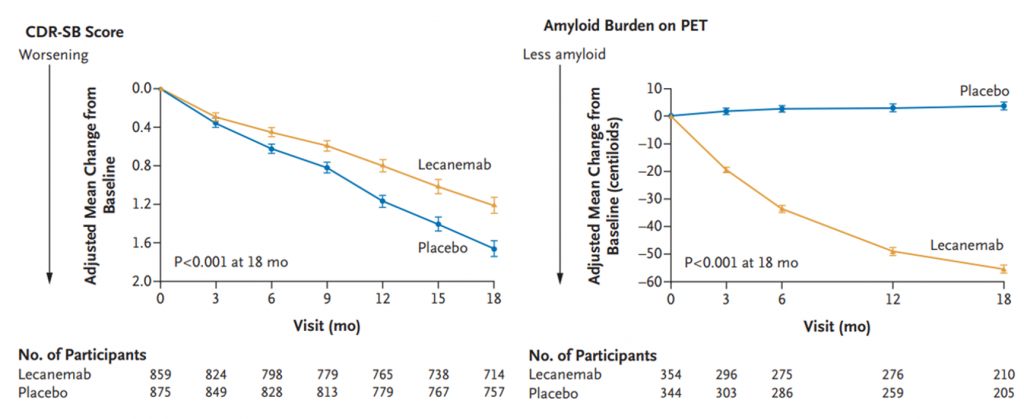
Published in the New England Journal of Medicine, Study 301 demonstrates lecanemab’s ability to delay Alzheimer’s progression and blunt the accumulation of amyloid plaques. This landmark 18-month, multicenter, double-blind, phase 3 clinical trial consisted of 1,795 patients with Alzheimer’s disease, half of which received a placebo or a dose of 10 mg/kg lecanemab once every two weeks via intravenous (vein) injection. To assess lecanemab’s effects on cognitive function, the trial’s investigators analyzed Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) scores – measurements from an array of tests utilized in Alzheimer’s clinical trials that look at the following domains: memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care.
CDR-SB scores range from 0-18, and higher scores signify greater impairment. Furthermore, a score of 0.5 to 6 is indicative of early-stage Alzheimer’s. At the beginning of the experiment, both placebo and lecanemab groups had a mean CDR-SB score of 3.2. However, after 18 months of treatment, scores from those treated with lecanemab rose by 1.21 while those treated with placebo rose by 1.66, demonstrating that lecanemab significantly delays Alzheimer’s-induced cognitive decline.
Swanson and colleagues proceeded to measure changes in amyloid plaque buildup using the Centiloid scale, a standardized measurement for amyloid. For reference, a score of 30 centiloids and below is considered to be the threshold for amyloid positivity, and the baseline measurements for the placebo and lecanemab groups were 75.3 and 77.92 centiloids, respectively. Lecanemab-treated patients, after 18 months, exhibited amyloid levels well below the threshold for amyloid positivity, while placebo-treated patients remained well above this threshold.
Notably, the brains of patients treated with lecanemab also displayed drastically lower levels of markers of neurodegeneration and neuroinflammation, suggesting that lecanemab successfully protects against Alzheimer’s-induced brain deterioration. Collectively, the trial’s findings support the use of lecanemb to thwart Alzheimer’s progression.
Possible Side Effects and Cost of Treatment
Although the trial’s findings are quite promising, it’s important to highlight lecanemab’s potential adverse effects following treatment. The investigators identified headaches and infusion-related reactions (fever, chills, and nausea) as the primary side effects. However, one of the more severe and prevalent (14% of patients) side effects following treatment was amyloid-related imaging abnormalities (ARIA), which can trigger swelling and bleeding on the surface of the brain. What’s more, ARIA can increase the risk of seizures and neurological dysfunction and in some cases, can lead to death.
Interestingly, the trial’s research team found that patients carrying the gene ApoE ε4 are more at risk of experiencing ARIA after treatment. More specifically, ARIA incidence is higher in homozygous carriers, individuals who inherited the ApoE ε4 gene from both parents. Because of this, the investigators suggest that doctors test for this gene prior to treatment. Equally, the investigators support the need for future experiments to further clarify lecanemab’s long-term effects on Alzheimer’s patients.
As a novel therapeutic drug, one might expect the cost of treatment to be extraordinarily high. And they are correct. The cost of yearly treatment is expected to be around $26,500. However, Dr. Greg Jicha, M.D., Ph.D., director of clinical trials at Sanders-Brown states, “Medicare has already made it quite clear that with FDA full approval, they are going to cover the medicine. Now people do need to be aware that there may still be a copay, your standard Medicare copay, especially if you do not have a Medicare supplemental plan that covers your copay. So that’s very, very important. Typically, if Medicare covers it, Medicaid covers it, and almost every third-party insurer will cover it.”