Key Points:
- A new mouse model for aging has been developed by inducing epigenetic changes.
- These mice display many features of aging, including memory deficits, reduced strength, and reduced endurance.
- The epigenetic changes can be reversed using cellular reprogramming — a technique used to revert cells to a younger state.
Not all our DNA is accessible to the machinery of our cells, and for good reason. We don’t want our muscle cells to access genes meant for immune cells, and we don’t want our skin cells to access the blueprint for neurons. Otherwise, we may have a cell identity crisis, whereby muscle cells begin converting to immune cells and skin cells transform into neurons. However, a new study suggests this change in DNA accessibility may cause aging.
“While DNA can be viewed as the body’s hardware, the epigenome is the software. Epigenes are proteins and chemicals that sit like freckles on each gene, waiting to tell the gene “what to do, where to do it, and when to do it,” according to the National Human Genome Research Institute.
Researchers from Harvard Medical School and 16 other universities and institutions across the world, led by Dr. David Sinclair, report in Cell that loss of epigenetic information causes aging in mammals. To test the hypothesis that epigenetic changes cause aging, Yang and colleagues developed a mouse model with inducible epigenetic changes. The results showed that these mice were similar to naturally aged mice. Furthermore, the epigenetic changes could be reversed by cellular reprogramming.
“[These findings] will transform the way we view the process of aging and the way we approach the treatment of diseases associated with aging,” said the lead author of the study, Jae-Hyun Yang.
ICE Mice Model (Epigenetic) Aging
It was once thought that aging is caused by the accumulation of genetic mutations — changes to the DNA sequence — but this has been largely disproven. It is now hypothesized that aging is caused by aberrant epigenetic changes, seemingly driven by double-stranded DNA breaks (DSBs). It is estimated that up to fifty DSBs occur in each of our cells every day. To repair these DSBs, epigenetic modifying enzymes (such as sirtuins) are recruited to the site of damage.
“The cell panics, and proteins that normally would control the genes get distracted by having to go and repair the DNA,” explained Dr. David Sinclair, the senior author of the study. “Then they don’t all find their way back to where they started, so over time it’s like a Ping-Pong match, where the balls end up all over the floor.”
It is proposed that the relocating of epigenetic modifiers in response to DSBs causes loss of genetic information and subsequent aging. To model this, Yang and colleagues developed mice with inducible DSBs (without genetic mutations). These inducible changes to epigenome (ICE) mice display features of aging not seen in normal mice, such as reduced weight, hunched back, and thin, gray fur.
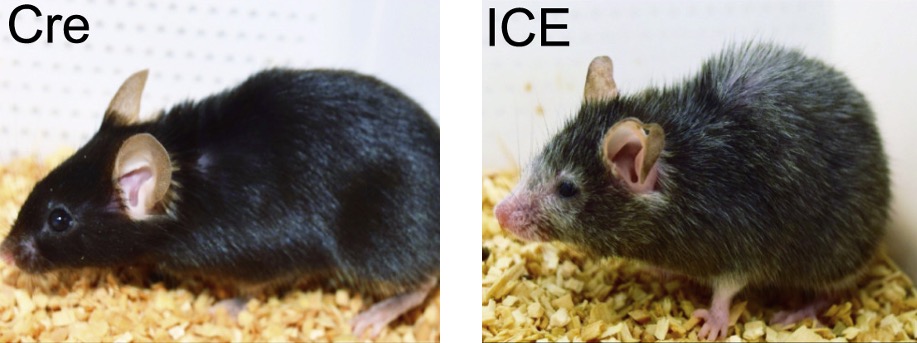
The hippocampus — the primary brain region responsible for consolidating memories — declines with aging. Using standard behavioral tests, Yang and colleagues found that ICE mice exhibit deficiencies in short-term and long-term memory, similar to that of naturally aged mice. These findings demonstrate that ICE mice model brain aging.
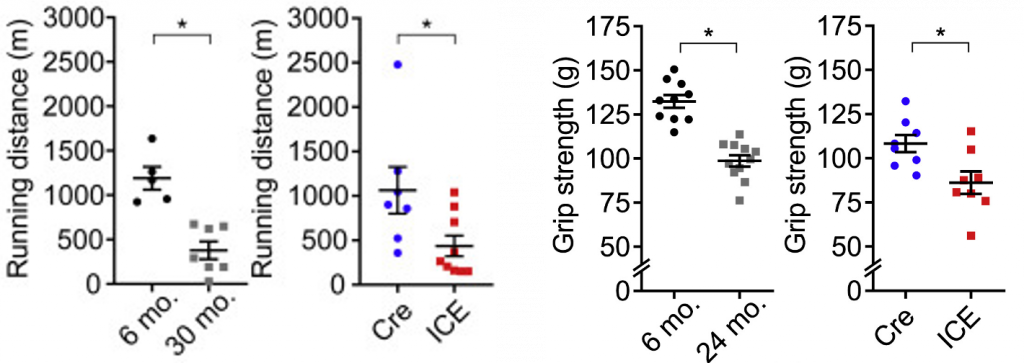
ICE mice also have less muscle mass, reduced running endurance, and reduced grip strength, similar to that of naturally aged mice. Moreover, their kidney tissue has less healthy waste-filtering glomeruli, a feature of kidney aging. Furthermore, based on biological clock calculations, the ICE mice age 50% faster. This, along with other pathological and molecular aging indicators, suggest that ICE mice accurately model aging.
Epigenetic Aging Can Be Reversed
Cellular reprogramming is a new technique that has been shown to increase the lifespan of naturally aged and prematurely aged mice while reversing indicators of aging. Reprogramming has also been utilized to reverse the age of human skin cells by thirty years and cure blindness in aged mice. This technique involves administering genes called Yamanaka factors, which cause cells to revert back to an earlier developmental stage.
It doesn’t matter if the body is 50 or 75, healthy or wracked with disease, Sinclair said. “Once that process has been triggered, “the body will then remember how to regenerate and will be young again.”
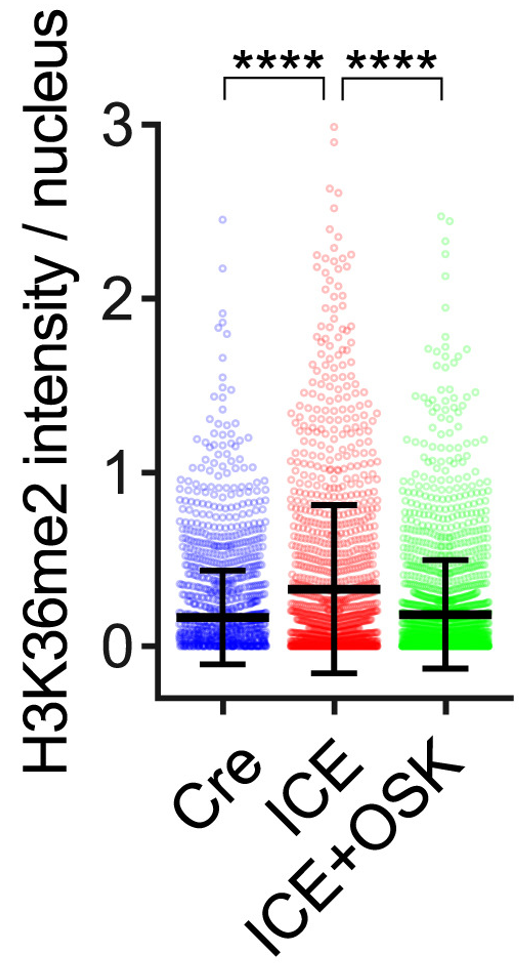
By administering reprogramming genes via adeno-associated virus (AAV) to the whole body of ICE mice for five weeks, Yang and colleagues improved aging markers in both kidney and muscle tissues. In the case of muscle, this marker is called H3K36me2, which is normally increased in aged muscle. H3K36me2 represents an epigenetic change to the genome that alters access to specific genes.
A New Way to Think About Aging
The work of Yang and colleagues support the Relocalization of Chromatin Modifiers, and the Information Theory of Aging hypotheses, which propose that the relocation of epigenetic modifiers leads to the loss of information needed to retain cellular identity, ultimately causing the cellular changes that underly the aging process.
“We believe it’s a loss of information — a loss in the cell’s ability to read its original DNA so it forgets how to function — in much the same way an old computer may develop corrupted software. I call it the information theory of aging.” – Dr. David Sinclair.